Ch. 1-3, Study of Life, Science & Chemistry, Cells
Ch 1.1: The Study of Life
Cell: basic unit of life
Organism: living individual consisting of one or more cells.
- Cells have an outer membrane to keep it together
- Contains DNA to produce proteins to carry out functions in tissues, organs, and organ systems
5 qualities are the scientific definition for life, which require the combination of all 5:
- Organization: atoms make up molecules -> cells -> tissues -> ...
- Energy Use: A kitten uses energy to go about its day
- Maintenance of internal consistency (homeostasis): A thermometer uses a temperature sensor to know when to heat or cool the room
- Reproduction, Growth, and Development: An acorn grows into a seedling, and at maturity sexually produces more acorns
- Evolution: Bacteria evolve against antibiotics.
Most scientists agree that viruses are not alive, because they do not grow, use energy on their own, nor maintain homeostasis. However, they do have a protein shell surrounding a small part of DNA. But this is not the structure of a cell, and thus it's even harder to say that it's alive.
A: Life is Organized
atoms -> molecules -> organelles -> cell -> tissue -> organ -> organ system -> organism -> population -> community -> ecosystem -> biosphere
A way to memorize this is the anagram AMOCTOOOPCEB. You can try to separate these out how you see fit.
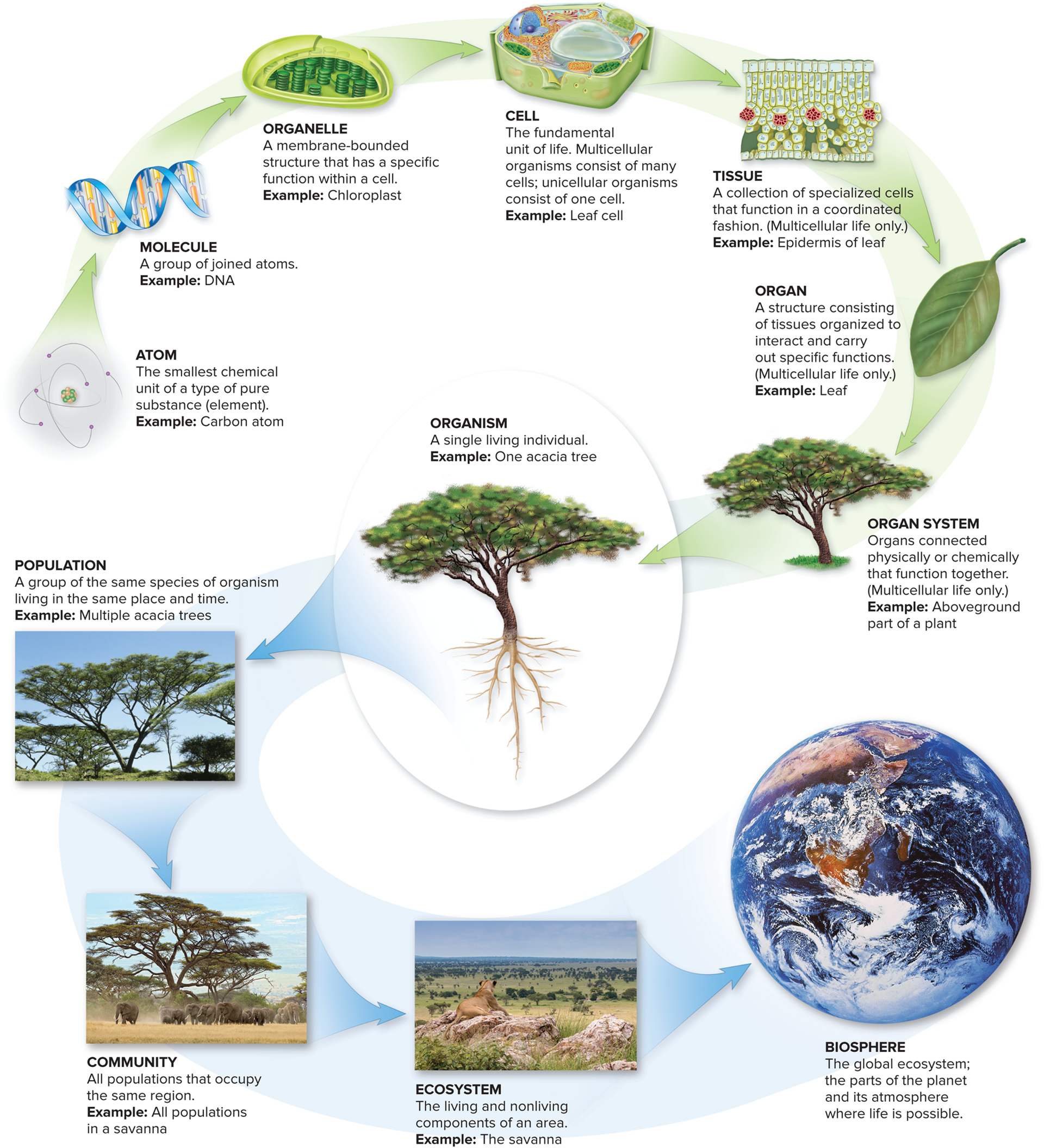
But importantly emergent properties, new functions that arise from interactions among a system's components, are brought out from the structures of these beings. For instance, brain cells use their structure to interact with other brain cells in order to allow humans to have a memory.
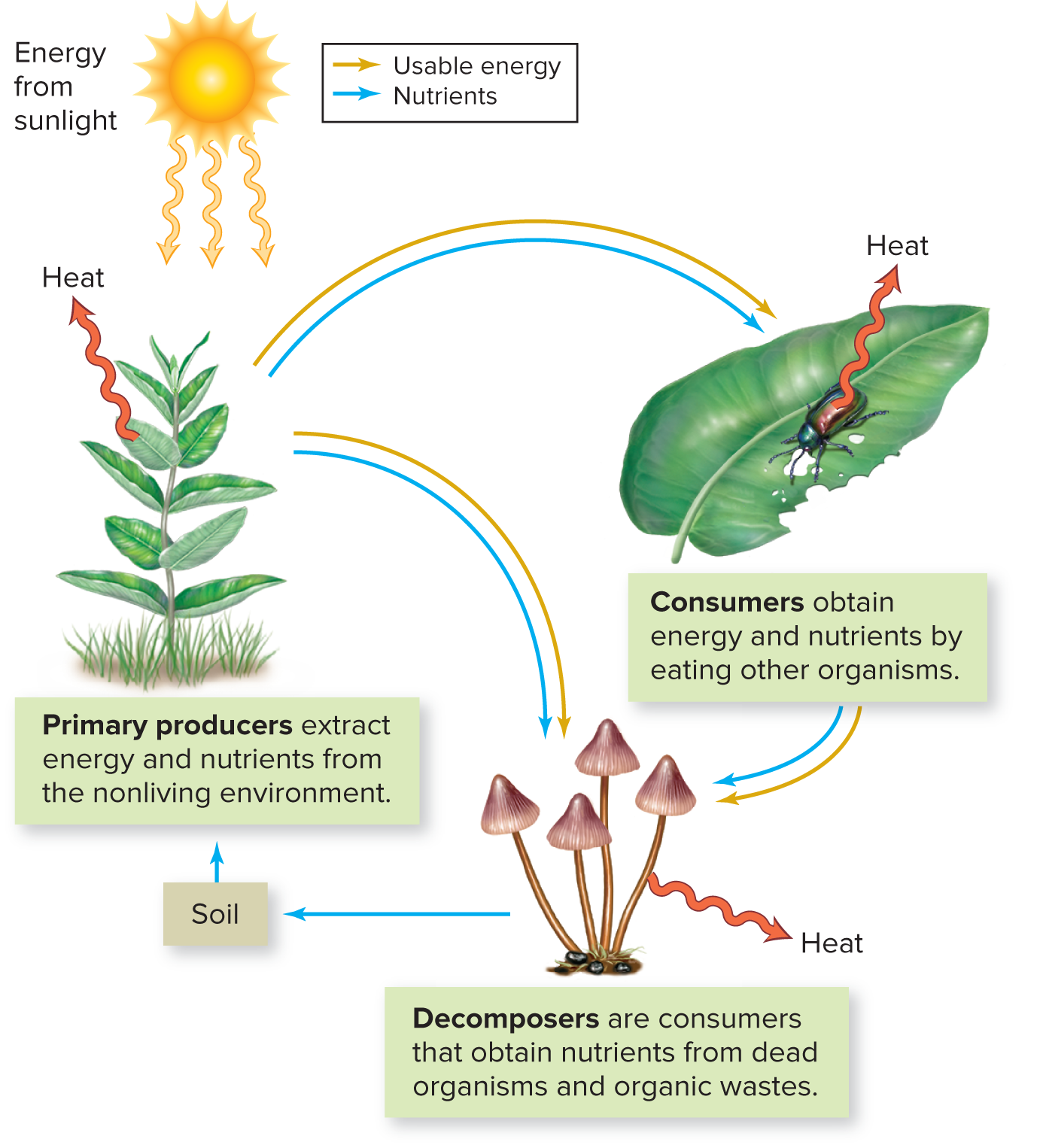
B: Life Requires Energy
Primary Producers (autotrophs) extract their nutrients from nonliving sources, ie: plants and microbes. Consumers (heterotrophs) get energy by eating other organisms. Decomposers get their energy from dead organisms (either producers or consumers). Heat is always lost when energy transfer occurs.
C: Homeostasis
Homeostasis is the state of internal consistency. One might shiver to increase their internal body heat in order to stay alive, or sweat to bring that temperature down. As long as one can both detect when changes need to occur, then have mechanisms react to that change, then you can maintain homeostasis.
D: Life Reproduces, Grows, and Develops
There's asexual reproduction, where genetic information comes from one parent and thus all the offspring are identical. Then there's sexual reproduction where genetic material from two parents unites to make a unique offspring, with inherited traits from both parents.
Sexual reproduction is beneficial in changing environments, as evolution allows successful genes to survive over generations. Development entails not only the growth but also the other changes that occur as an organism matures. This is different than growth, which is just a physical size increase by cell division or whatnot.
E: Life Evolves
An adaptation is an inherited characteristic or behavior that allows an organism to survive and reproduce successfully in its environment. This occurs by natural selection, the enhanced reproductive success of certain individuals from a population based on inherited characteristics.
Natural selection is one mechanism of evolution, which is a change in genetic makeup of a population over multiple generations.
Ch 1.2: The Tree of Life Includes Three Main Branches
Taxonomy is the science of classification. Biologists study types of organisms, or species, by probably evolutionary relationships. A genus consists of closely related species.
The two broadest taxonomic levels are domain and kingdom. The three domains of life are:
- Archaea: one of two domains of prokaryotes (DNA is free in the cell and not confined to the nucleus)
- Bacteria: one of two domains of prokaryotes (DNA is largely different in sequence to Archaea)
- Eukarya: DNA is within the nucleus
- Protista (multiple kingdoms): Unicellular or multicellular autotrophs (producers) or heterotrophs (consumers)
- Kingdom Animalia: Heterotrophs (by ingestion)
- Kingdom Fungi: heterotrophs (by external digestion)
- Kingdom Planae: autotrophs
Then the species in each domain are divided into kingdoms, then divided into multiple phyla, then into:
- class
- order
- family
- genus
- species
The scientific name comes from using (genus, species), such as in homo sapien.
Ch 1.3: The Scientific Method
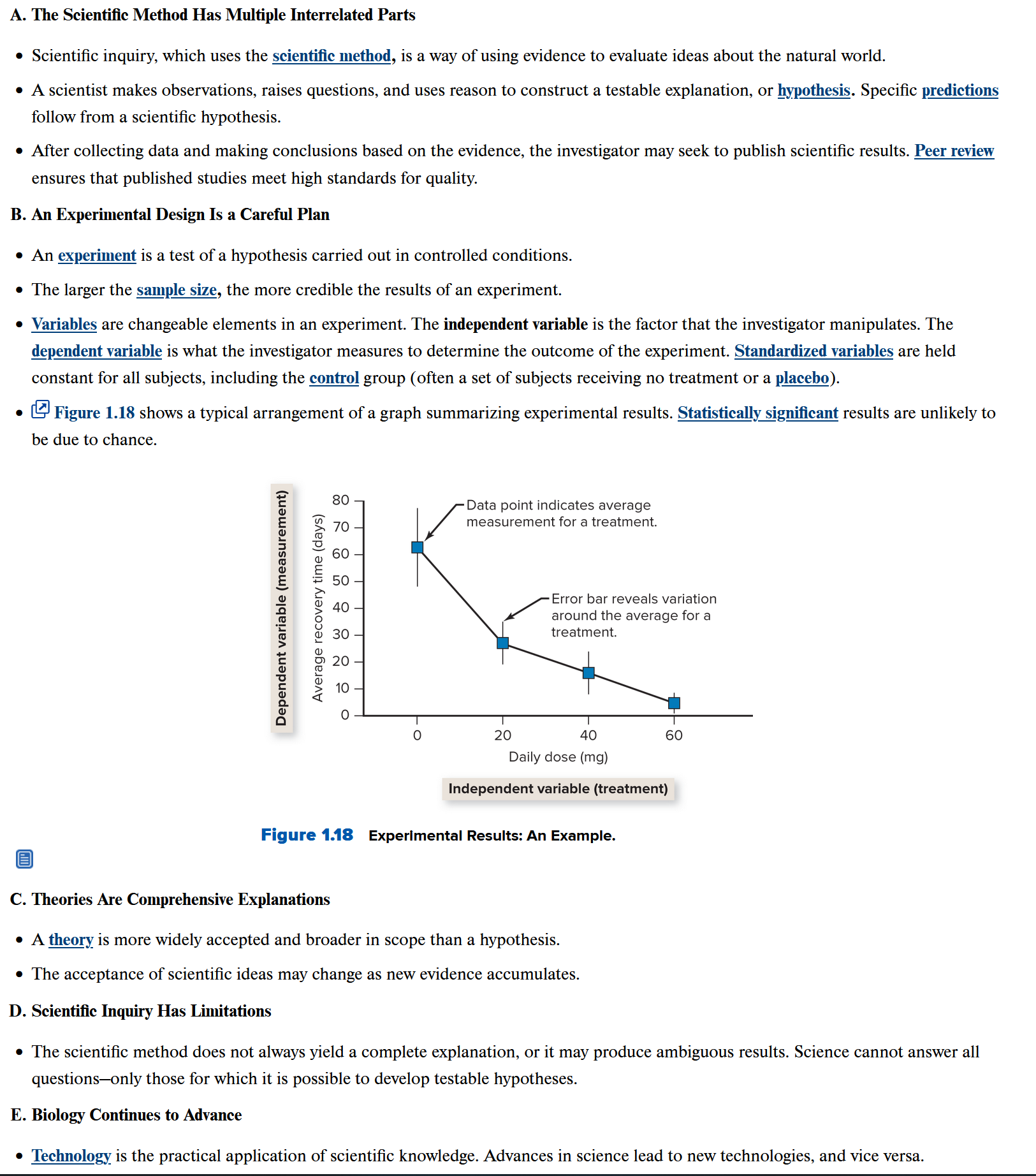
Ch 2.1: Atoms Make Up All Matter
All substances contain matter and energy, the ability to do work. All matter can be broken down into pure substances called elements.
Bulk elements are essential to life in large quantities. The most abundant are C, H, O, and N. The trace elements are needed in smaller amounts and are S and P, along with others.
An atom is the smallest unit of an elements, with positively charged protons and neutral neutrons to form the nucleus. The negatively charged electrons surround the nucleus. Elements are organized via the periodic table by their atomic number, or the number of protons they have. An ion is an atom that gains/loses electrons.
Isotopes of an element differ by the number of neutrons. A radioactive isotope is unstable. An element's atomic weight reflects the average mass number of all isotopes, weighted by the proportions in which they naturally occur.
Ch 2.2: Chemical Bonds Link Atoms
A molecule is two or more atoms joined together. If they are from different elements, they are a compound.
Electrons move constantly; they are most likely to occur in volumes of space called orbitals, which are grouped into energy shells. An atom's tendency to fill its valence shell drives it to form chemical bonds with other atoms. The more electronegative an atom, the more strongly it attracts electrons, and determines the type of bond formed:
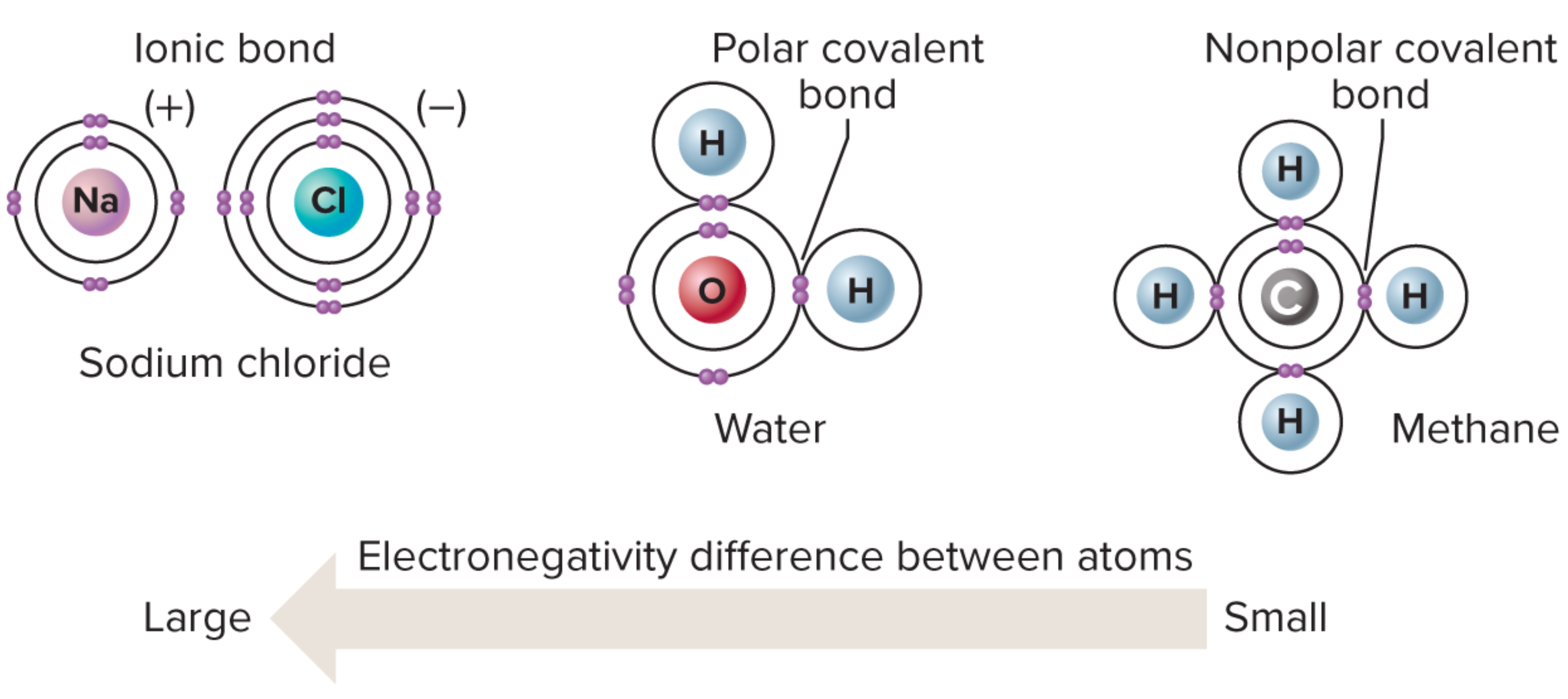
In an ionic bond, the attraction is so great that one electronegative atom strips an electron (or more) from the other. In a covalent bond, electrons are shared. In a nonpolar covalent bond, these electrons are shared equally. If one atom is more electronegative than the other, a polar covalent bond forms, giving partial charges
Ch 2.3: Water is Essential to Life
Water has many important properties:
- Water molecules stick to each other (cohesion) and to other substances (adhesion)
- A solution consists of a solute dissolved in a solvent, and water dissolves hydrophilic (polar and charged) substances but not hydrophobic (nonpolar) substances.
- Water regulates temperature in organisms because it resists temperature change and evaporation. Large bodies of water help keep coastal climates mild, for example.
- Water expands when it freezes, such as how ice floats when in water.
- In a chemical reaction, the products are different than the reactants, and most biochemical reactions occur in a watery solution, using water as a reactant (and/or sometimes is a catalyst)
Ch 2.4: Cells Have an Optimum pH
In pure water, the concentrations of
Buffers consist of weak acid-base pairs that maintain the optimal pH ranges of body fluids by releasing or consuming
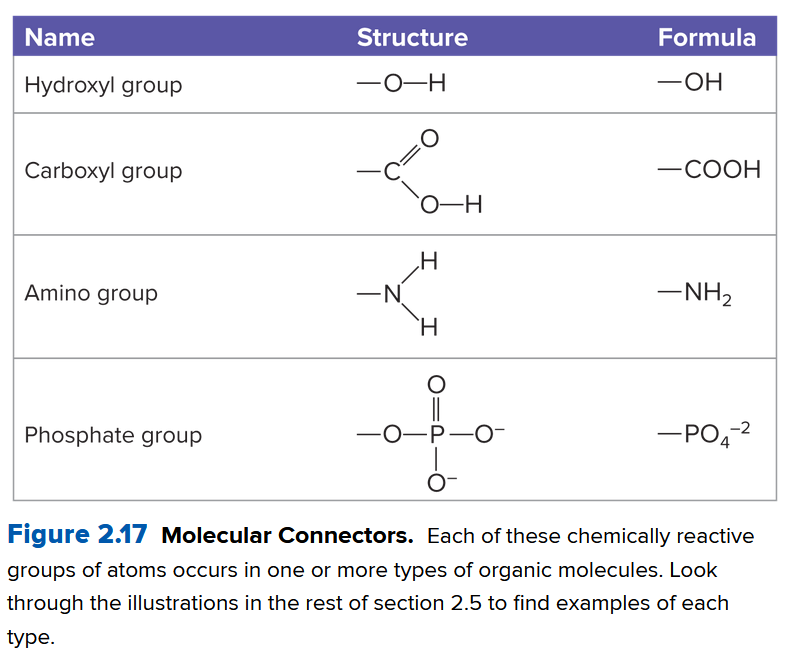
Ch 2.5: Cells Contain Four Major Types of Organic Molecules
Many organic molecules (molecules containing carbon and hydrogen) consist of small subunits called monomers, linking together to form polymers. Dehydration synthesis is the chemical process that joins monomers together releasing a water molecule:
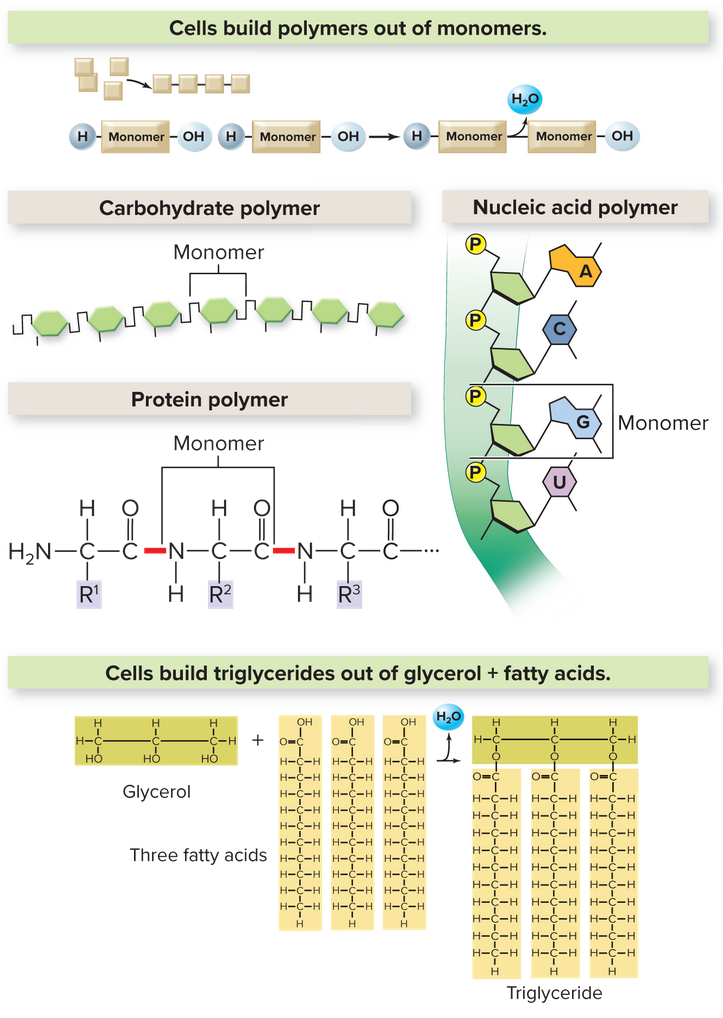
Here, a protein called an enzyme removes an
The hydrolysis reaction does the opposite, using water to break polymers into monomers. We talk about the different types of polymers below
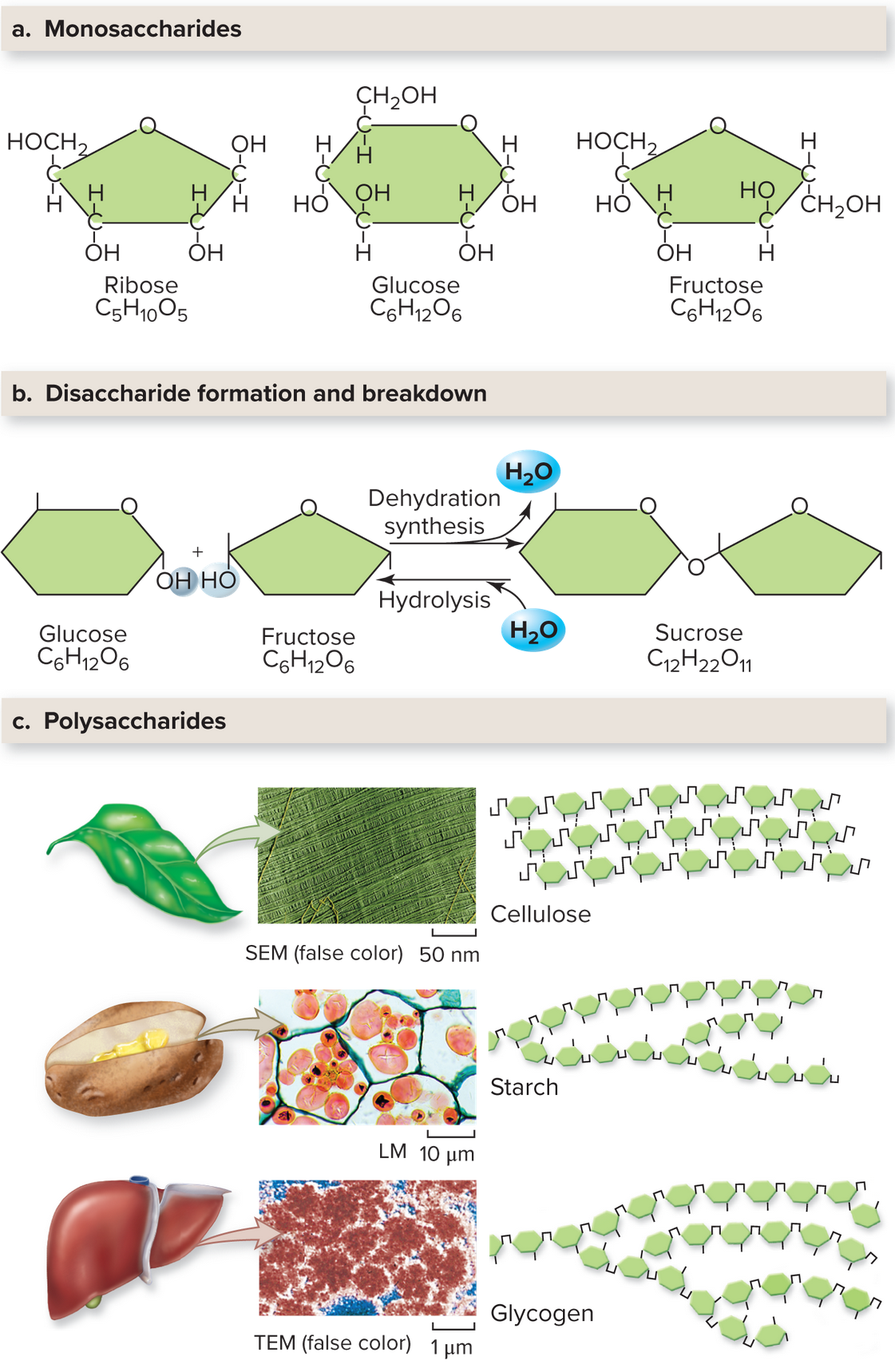
Carbohydrates Include Simple Sugars and Polysaccharides
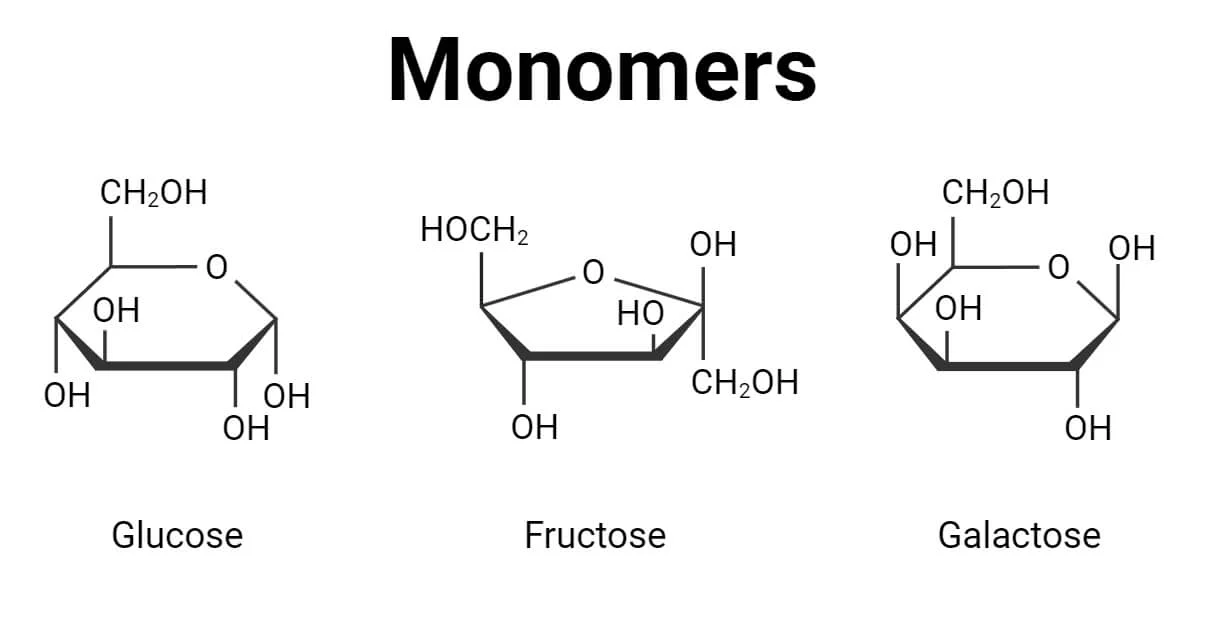
Carbohydrates are compounds consisting of carbon, hydrogen, and oxygen in proportions 1:2:1. Monosaccharides are single-ring sugars like glucose. Two bonded monosaccharides form a disaccharide. These simple sugars provide quick energy. Polysaccharides rather are complex carbohydrates consisting of hundreds of monosaccharides, helping provide support and to store energy.
Proteins are Complex and Highly Versatile
Proteins consist of amino acids, each composed of a central carbon atom bonded to a hydrogen atom, a carboxyl group (see Ch. 1-3, Study of Life, Science & Chemistry, Cells#^b16e9e), an amino group (
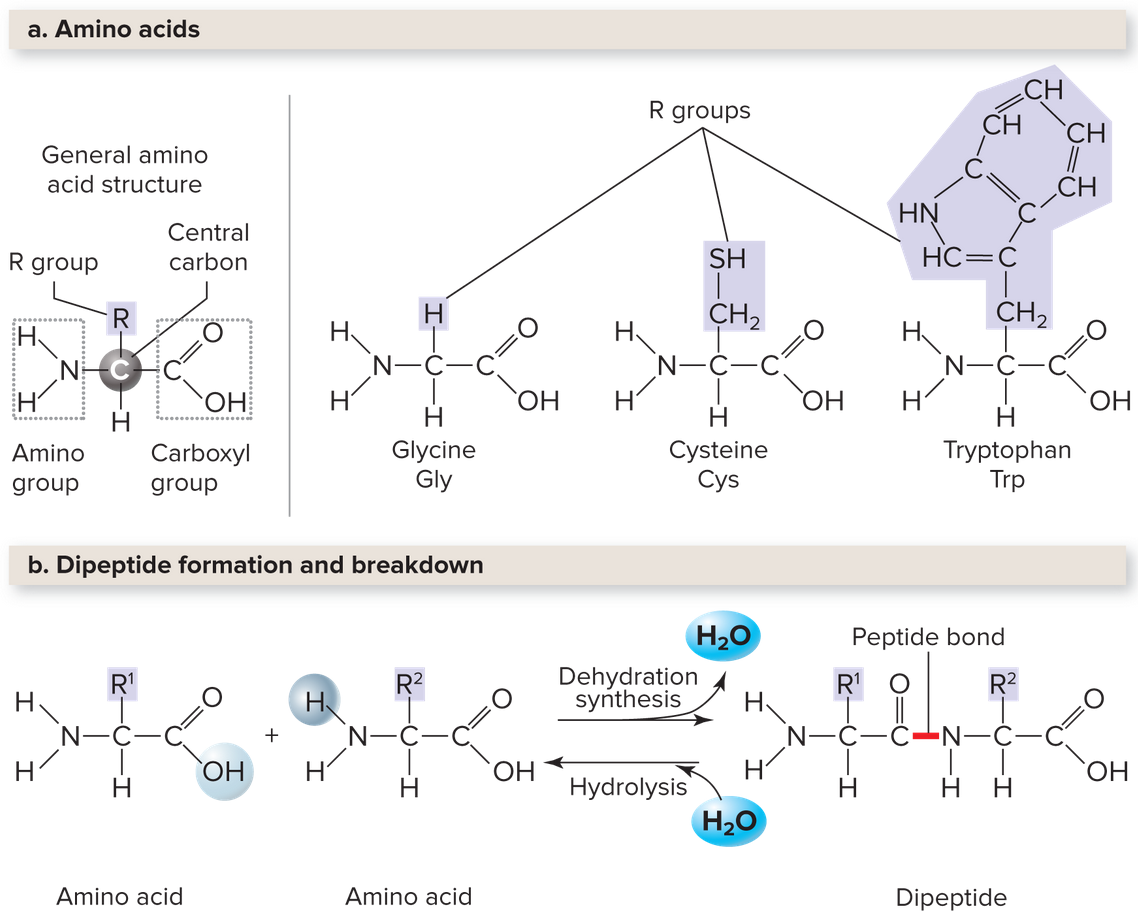
Each R group distinguish the amino acids that are essentially the monomers for proteins. Mixing and matching the R groups rise to an endless diversity of unique proteins. Instead of polymeric bonds, we call these single unit bonds a peptide bond that links each amino acid to one another. Two amino acids linked form a dipeptide, and three make tripeptide. Long chains form polypeptides, and once formed into a functional shape is called a protein.
Humans can make some of these protein from starches they eat, but a lot have to come from protein-rich foods we digest.
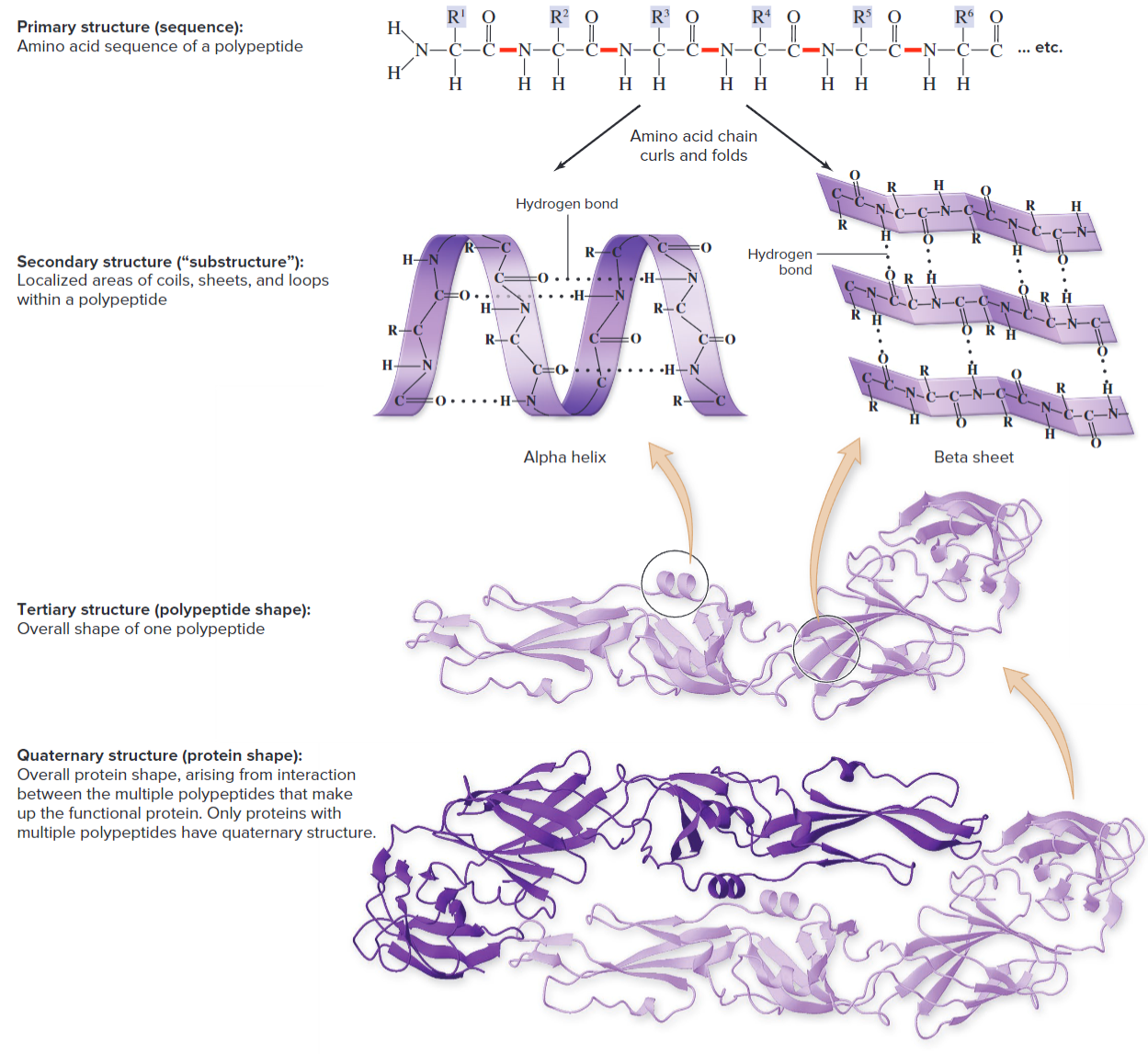
Unlike polysaccharides, proteins don't exist as long chains inside cells, but rather as 3D folds via their order and kinds of amino acids. There's 4 different types of structure:
- Primary structure: The amino acid sequence of a polypeptide chain. Determines all subsequent structure levels.
- Secondary structure: A substructure with defined shape, resulting from hydrogen bonds between parts of the polypeptide, creating coils/loops/etc.
- Tertiary structure: Overall shape of a polypeptide, arising primarily between R groups and water.
- Quaternary structure: Protein shape, multiple tertiary structures. Only in proteins with multiple polypeptides.
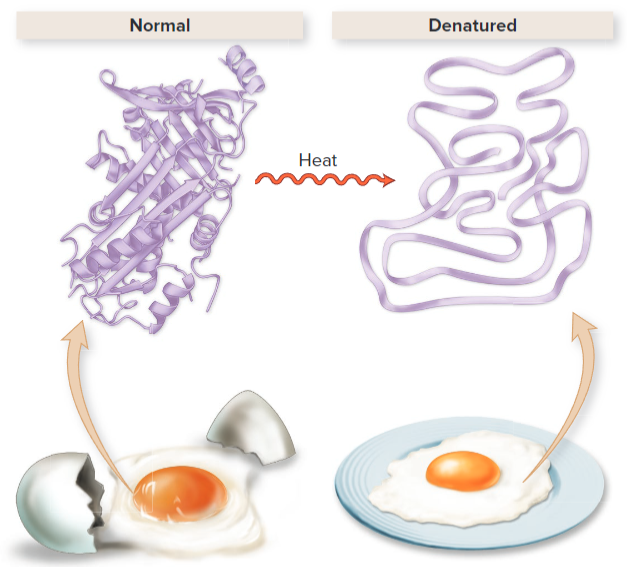
The structure of a protein dictates what job/abilities it can do! Hence, things like pH and heat that alter their shape have drastic consequences, called denaturing the protein. You cannot reverse the process of denaturing.
Nucleic Acids Store and Transmit Genetic Information
A nucleic acid is a polymer consisting of monomers called nucleotides. There's two types, DNA and RNA. Each nucleotide consists of:
- A 5-carbon (pentagonal) sugar, deoxyribose in DNA and ribose in RNA
- A phosphate group
- nitrogenous base, such as Adenine, Guanine, Thymine, Cytosine, or Uracil. DNA has all but Uracil, while RNA has all but Thymine.
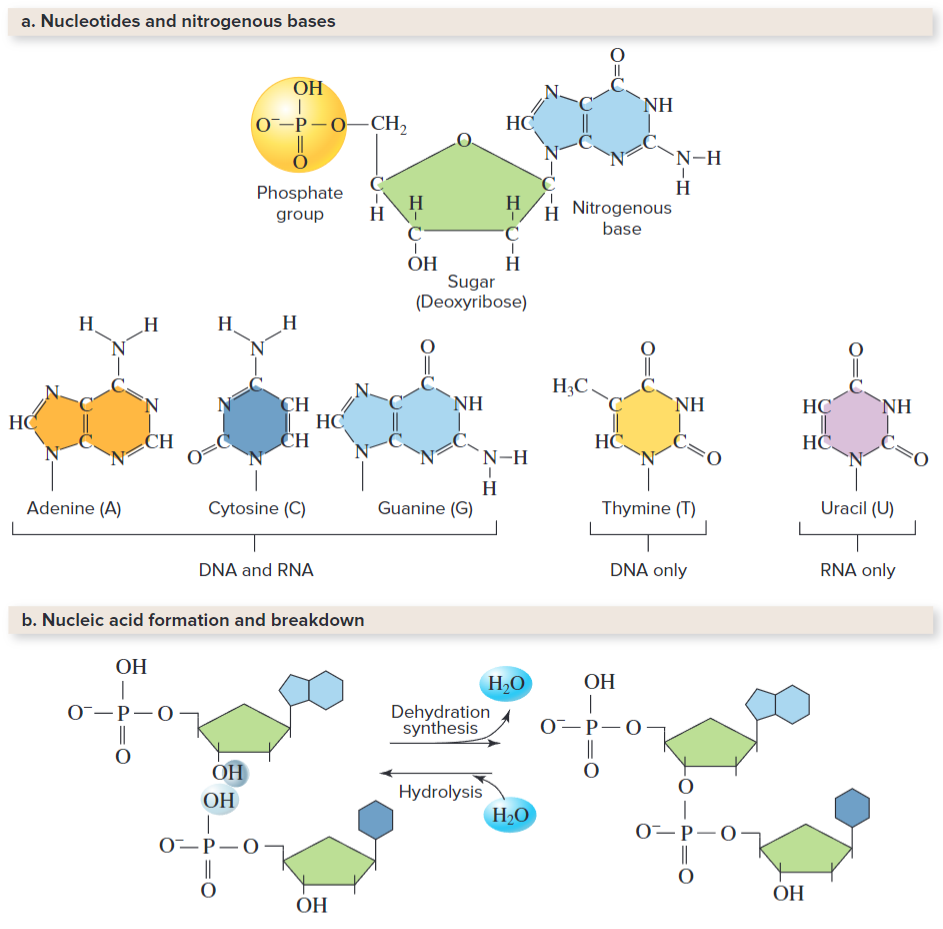
Hydrogen bonds hold alternating pairs with each other (A with T, C with G). Unlike DNA, RNA is single stranded, and RNA helps enable cells to use protein-encoded information from the DNA. It also carries ATP (energy) that cells use in other biological functions.
Lipids Are Hydrophobic and Energy Rich
Lipids are organic compounds with one property in common, they don't dissolve in water. They are non-polar due to their nonpolar carbon-carbon and carbon-hydrogen bonds.
Triglycerides
A triglyceride (fat) includes three fatty acids, chains of almost entirely carbon and hydrogen. They are bounded to glycerol that acts as the backbone of it. 3 fatty acids are per glycerol molecule, giving 3 molecules of water during dehydration synthesis (or using 3 during hydrolysis).
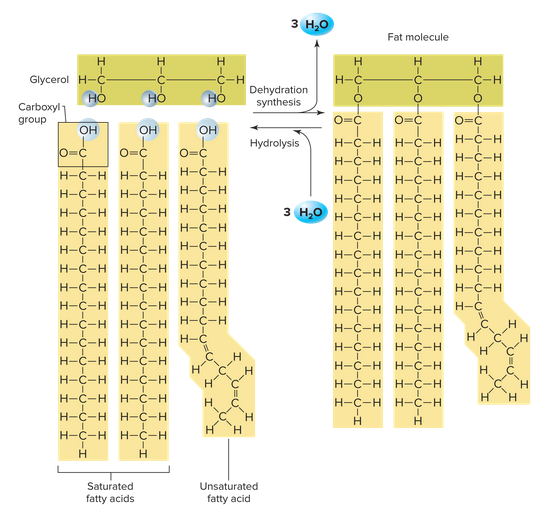
A saturated fatty acid contains all the hydrogens it possibly can. These are really bad, so it best to have low amounts of saturated fat in your diet. An unsaturated fatty acid has at least one double bond between carbon atoms. Things like olive oil, fish , nuts, and avocados have these, and are healthier than their saturated counterparts.
Butter is solid at room temperature because it's more saturated than something like olive oil, which is liquid. As a result, then there more of a rigid structure in saturated fats, making them more solid at room temperature:
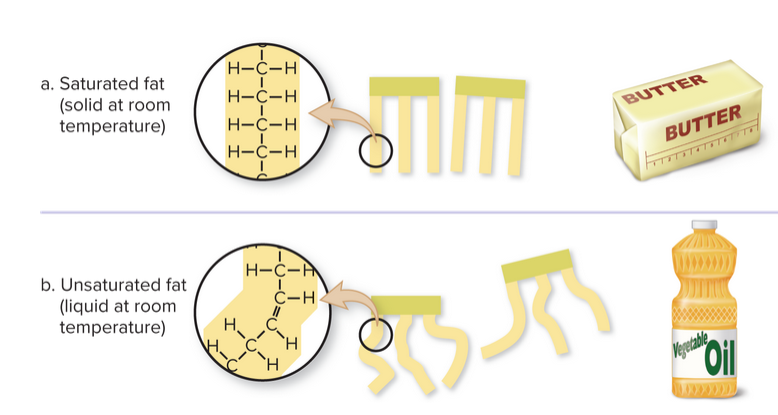
Fat helps mainly with energy storage, and are required for growth as well as some vitamins and minerals (micronutrients).
Steroids
Steroids are lipids with 4 interconnected carbon rings. Cholesterol is an example of a steroid. It's used as a starting material to make other lipids.
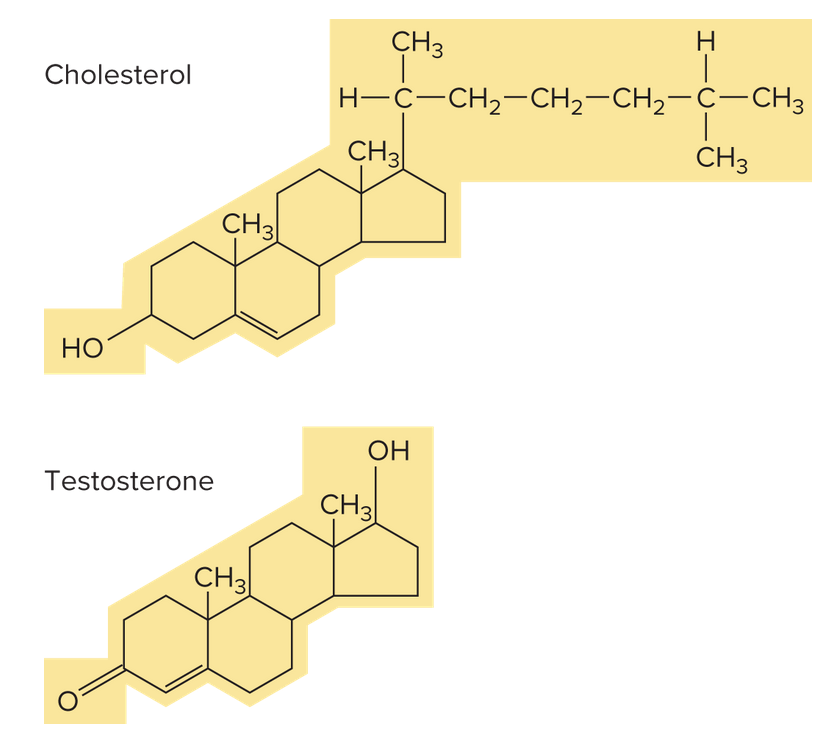
The liver produces cholesterol that the animal body needs, so really diets with any cholesterol should be limited as much as possible.
Ch 3.1: Cells are the Units of Life
We'll talk about cells, the smallest units of life that can function independently. In 1839, German biologists Mathias J. Schleiden and Theodor Schwann formulated the cell theory that says:
- All organisms are made of one or more cells
- The cell is the fundamental unit of life
- All cells come from preexisting cells (added by Rudolf Virchow)
Below, we talk about the ranges and limitations of current microscopes used to observe cells:
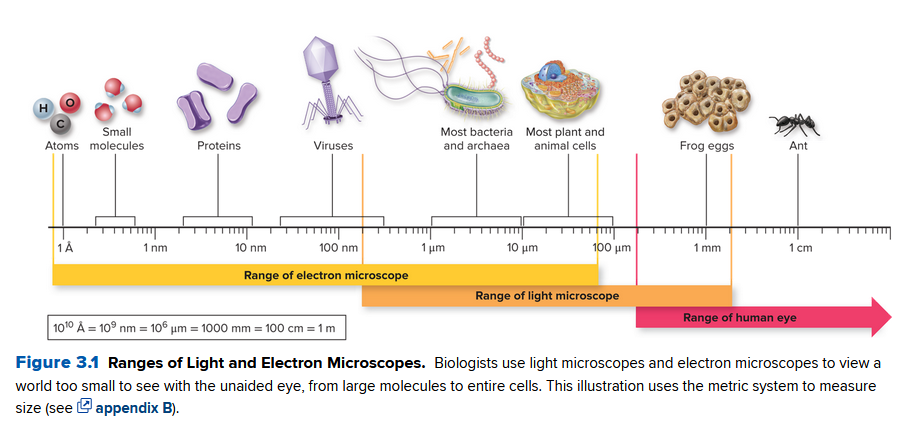
Light Microscopes
- Generate true-color views of living or preserved cells
- Two types: compound an d confocal
- Compound: uses two or more lenses to focus visible light through the specimen. The specimens present must be transparent
- Confocal: Sends a laser through the object.
Transmission/Scanning Electron Microscopes
- Sends a beam of electrons through the specimen
- Lower resolution than light microscopes
- Can reveal textures on the surface
- Specimens must be dead to avoid movement during measurement
- Only color
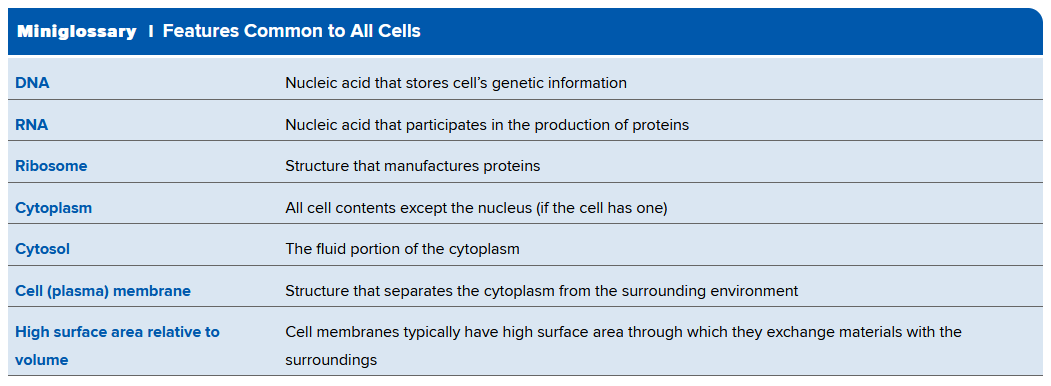
Common Cell Features
- All cells contain DNA, and similarly RNA to help produce proteins to do work
- Contain ribosomes: structures that manufacture proteins
- Cell Membrane: a lipid-rich boundary between the cell and its environment
- Membrane encloses cytoplasm, the watery mixture that occupies much of a cell's volume; in eukaryotic cells it consists of all materials between the nuclear envelope and the cell membrane
- Cytosol is the fluid portion of cytoplasm.
Some other facts:
- Cells avoid surface area, and thus are small in volume as
grows faster than as dimension grows. - A large
maximizes interactions with the environment, and is ideal.
Ch 3.2: Different Cell Types Characterize Life's Three Domains
Two categories of life:
- prokaryotes: simples and most ancient form of life that doesn't have a nucleus
- Bacteria
- Archaea
- eukaryotes: cells contain organelles, compartments that carry out special functions, such as the nucleus.
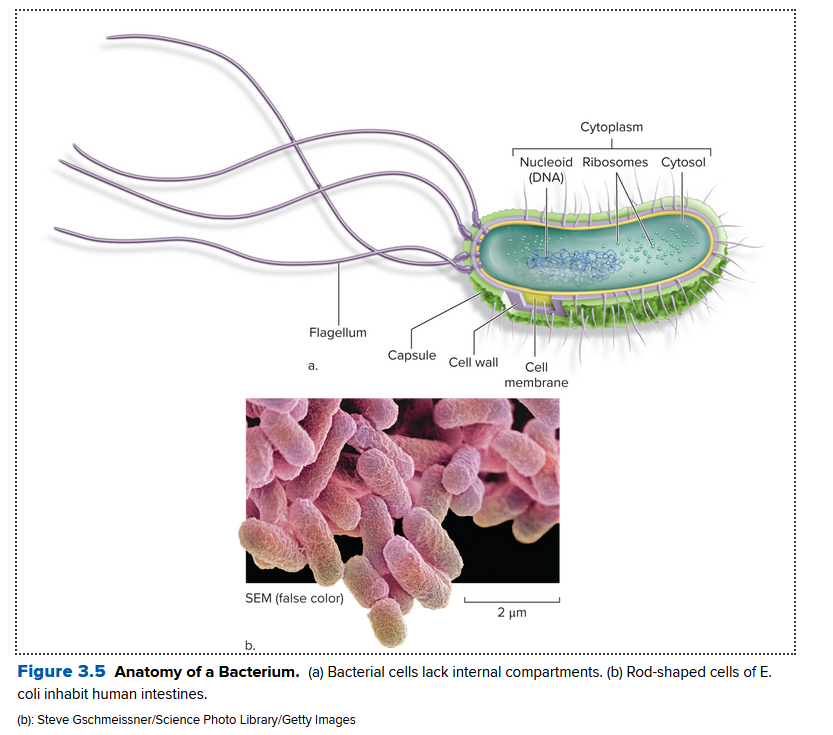
In a bacteria:
- nucleoid: area where the cell's circular DNA molecule congregates (not bound by a membrane)
- A cell wall surrounds the cell membrane
- Bacteria can swim via their flagella, a tail-like appendage
- are usually single celled
Archaea are similar to bacteria to what's described above. But they are built out of completely different biochemicals and have completely different DNA sequences. Archaea, by their DNA, are most closely related to Eukaryotes.
Domain Eukarya
Compare the two different cells for animals and plants below:
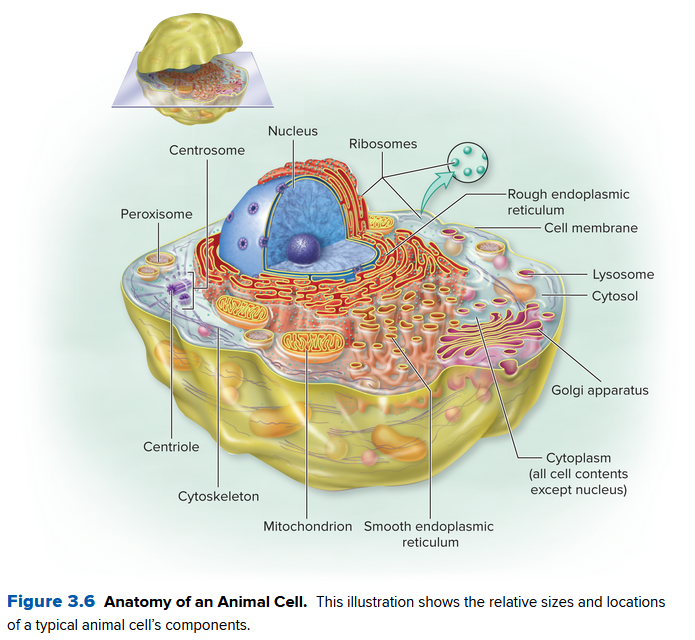
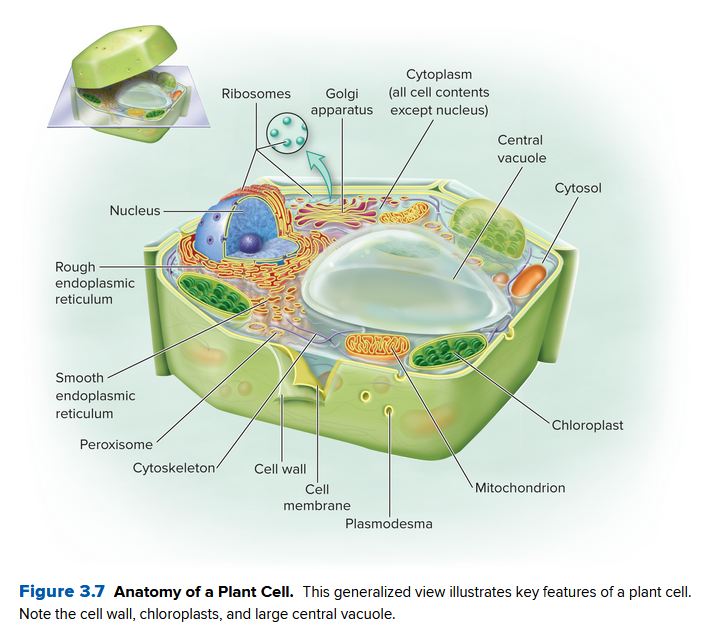
The only difference is a lack of chloroplasts and a cell wall in animal cells, and plant cells lack centrioles.
Eukaryotic cells are very big, normally orders of magnitude bigger than prokaryotic cells. Mainly:
- The cytoplasm of a eukaryotic cell is split into organelles, via cell internal membranes
Ch 3.3: A Membrane Separates Each Cell from Its Surroundings
The cell membrane:
- separates cytoplasm from the surroundings
- transports substances in and out of the cell
- enclose organelles
They are made of phospholipids: a triglyceride and phosphate group along with fatty acids:
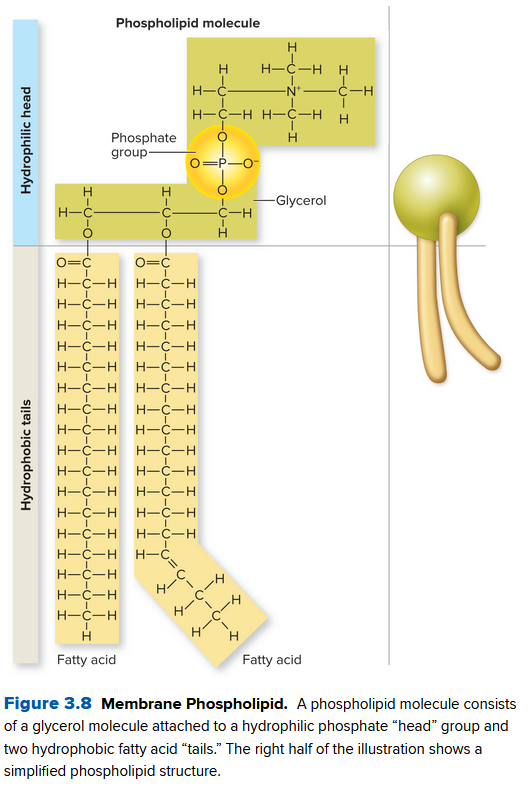
The head therefore is hydrophilic (attracted to water) and the tail is hydrophobic. They assemble in a phospholipid bilayer, a double layer of phospholipids.
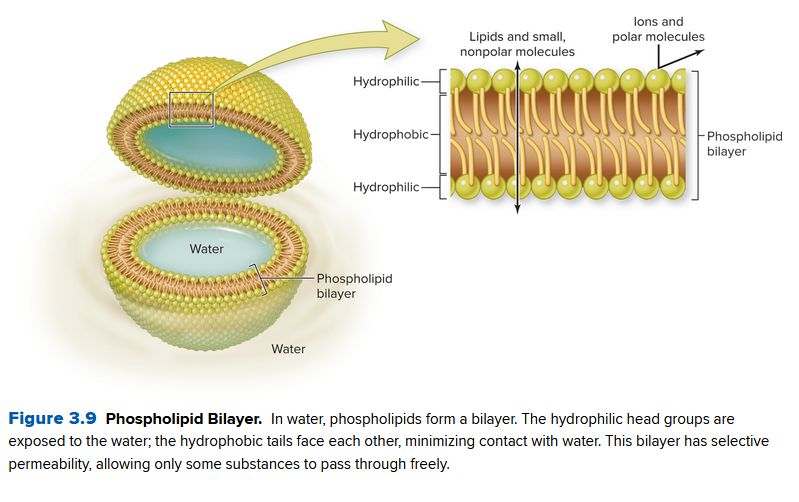
Since the middle is hydrophobic, there's selective permeability, filtering what molecules can pass through. Nonpolar molecules can freely pass, like
A membrane also contains proteins to let some of these through. A membrane is often called a fluid mosaic since the molecules drift laterally (within) the bilayer.
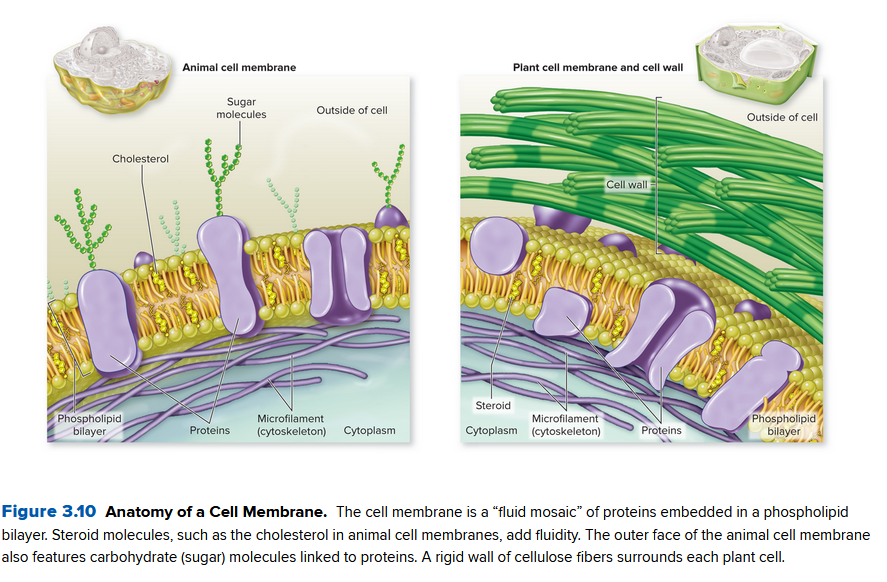
Some proteins span both sides, while some only face on one side of the membrane:
- Transport proteins: Transport proteins imbedded in the p. bilayer create passageways for ions, glucose, ... in or out of the cell
- Enzymes: Facilitate chemical reactions that otherwise would proceed too slowly to sustain life
- Recognition Proteins: Carbohydrates on the cell surface help the immune system realize it is its own cell
- Receptor proteins: Bind to molecules outside the cell and trigger an internal cell response
Ch 3.4: Eukaryotic Organelles Divide Labor
Organelles in eukaryotic cells all carry out different functions. They mainly:
- Keep related biochemicals and structures close together
- Coordinated endomembrane system: eukaryotic organelles that exchange materials in transport vesicles: small membranous spheres that transport materials in the cell.
The Nucleus, Endoplasmic Reticulum, and Golgi Interact to Secrete Substances
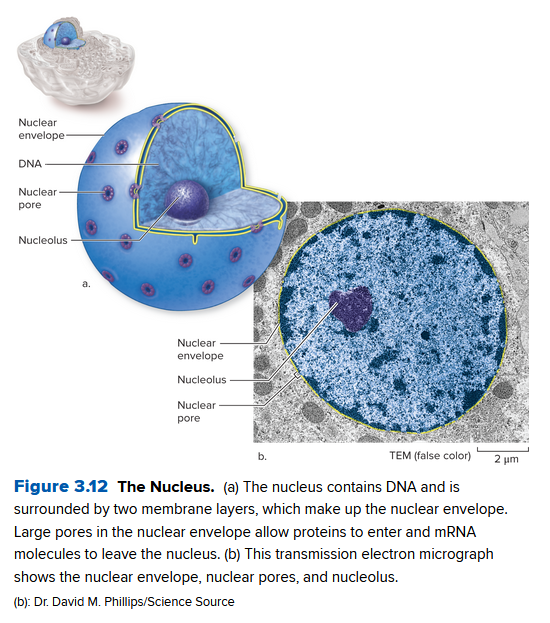
The nucleus:
- contains most of the DNA in the cell
- gives the recipes for each type of protein to make
- contains nuclear pores, holes in the double-membrane nuclear envelope that separates the nucleus from the cytoplasm
- mRNA copies the internal DNA and leaves the nucleus via these pores to help latter make these proteins
- Center nucleolus: dense spot that assembles components of ribosomes, where they leave the nucleus to become complete ribosomes.
The endoplasmic reticulum and golgi apparatus:
- mRNA leaving the nucleus binds to a ribosome to manufacture proteins
- the endoplasmic reticulum (ER) is a network of sacs and tubules composed of membranes, starting near the nuclear surface and winding outward
- Proteins near the edge of the ER takes the free ribosomes and mRNA to make proteins
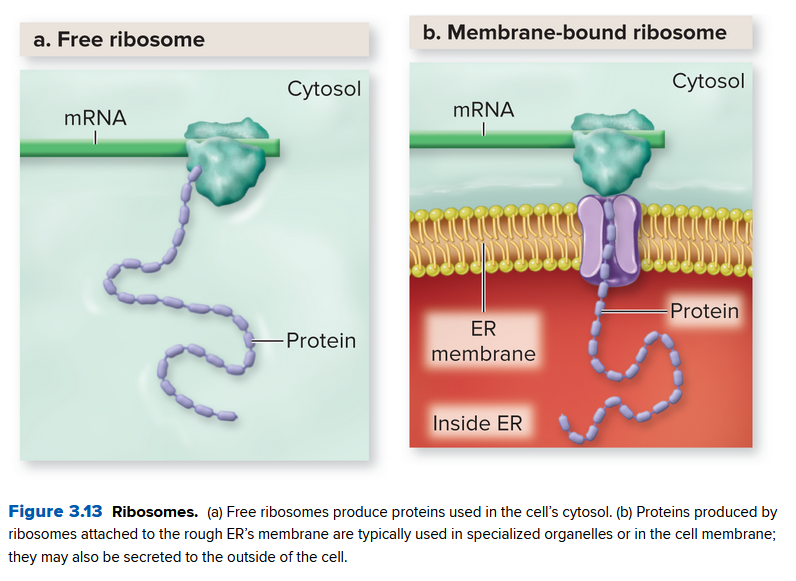
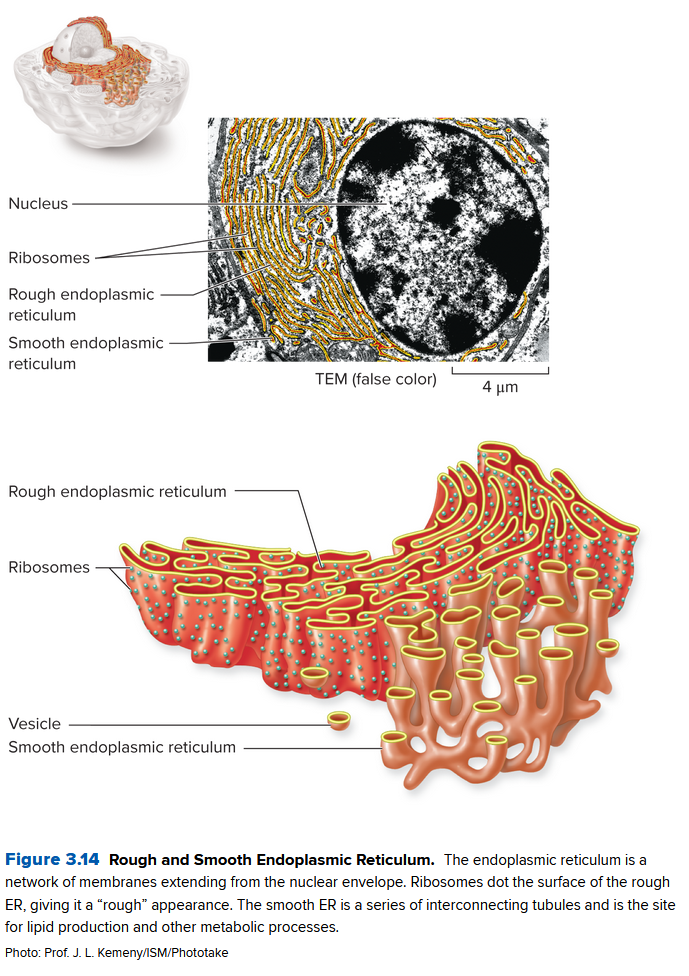
Here the rough endoplasmic reticulum synthesizes these proteins, while the smooth endoplasmic reticulum synthesizes lipids and does other metabolic processes.
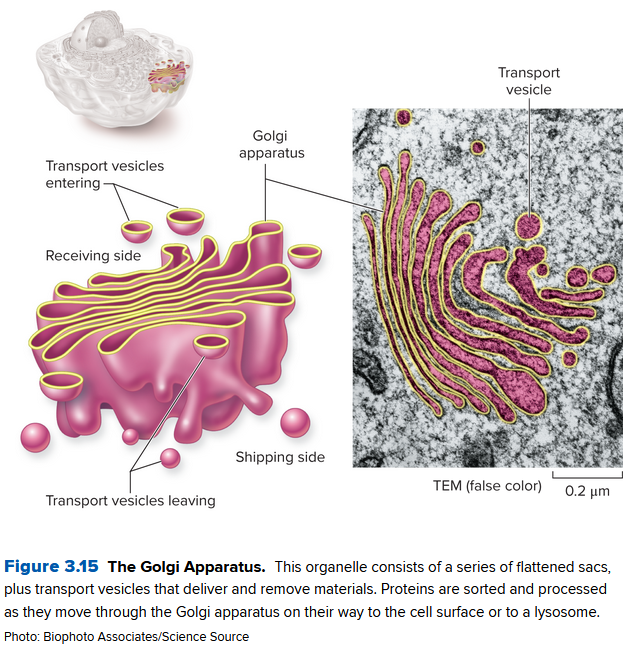
Vesicles take the lipids and proteins and go to the Golgi Apparatus, a stack of flat, membrane-enclosed sacs that packages cell products for export. They also have enzymes (recognition proteins) that help create the name tags that stop the immune system from deleting them from existence.
Lysosomes, Vacuoles, and Peroxisomes Are Cellular Digestion Centers
Lysosomes
Organelles that contain enzymes that dismantle and recycle food particles, captured bacteria, worn-out organelles, and debris. Their enzymes lyse (cut apart) their substances.
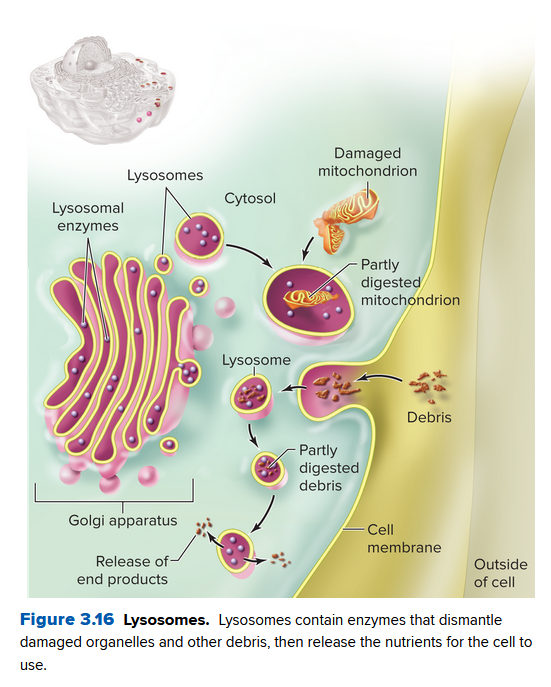
- The enzymes in lysosomes start from the rough ER.
- The GA detects these enzymes and packages them into vesicles that become lysosomes
- These attach to internal or external materials to break them down (recycle, or destroy them)
- They are acidic internally, helping the dissolving process.
Vacuoles
Plant cells usually lack lysosomes, but have vacuoles that essentially do the same thing as lysosomes. It also takes up most of the space in plants.
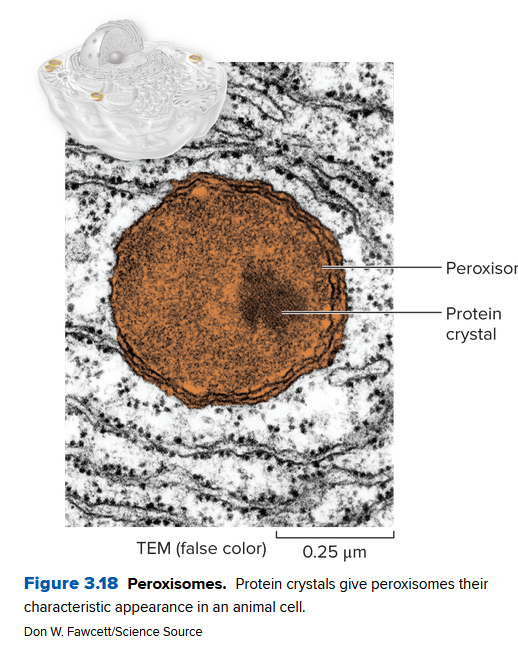
Peroxisomes
All euk. cells contain peroxisomes, organelles containing several types of enzymes that dispose of toxic substances. They come from the ER (not the Golgi) and contain different enzymes. Usually they're distinct by their condensed protein crystals:
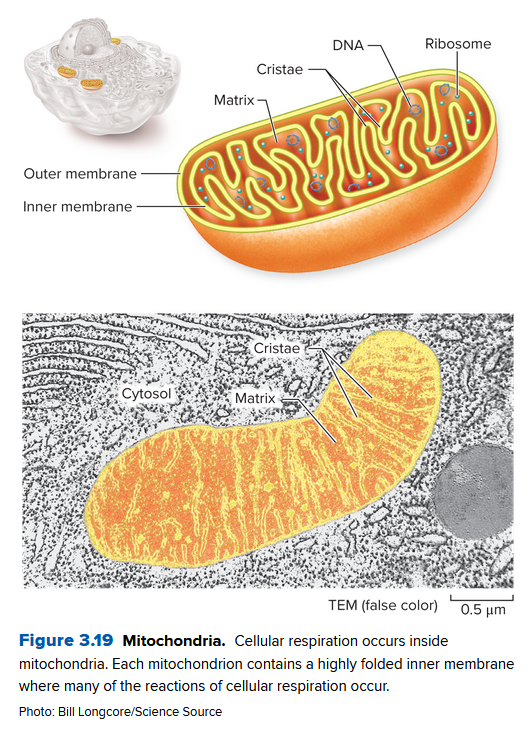
Mitochondria Extract Energy from Nutrients
Mitochondria are organelles that use cellular respiration to extract energy from food. Except for a few protists, all euk. cells have mitochondria. They contain:
- an outer membrane:
- inner membrane (with mitochondrial matrix): contains DNA that encodes proteins needed to help break down the food
- Cristae: folds of the inner membrane, adding needed surface area to the organelle
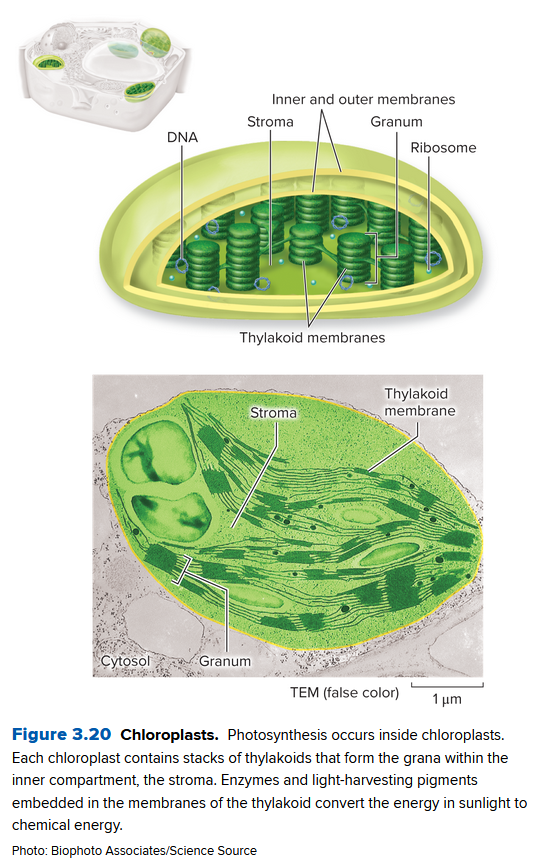
Photosynthesis Occurs in Chloroplasts
The chloroplast is the site of photosynthesis in eukaryotes:
- Double membrane layer encloses the stroma: a third membrane layer
- Thylakoid Membranes form structures of stacked "pancake" grana
- Photosynthetic pigments like chlorophyll are embedded in thylakoid membranes
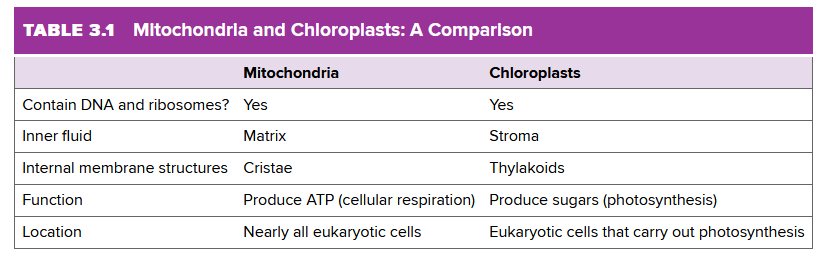
They're essentially the plant-version of mitochondria, except for some specific differences:
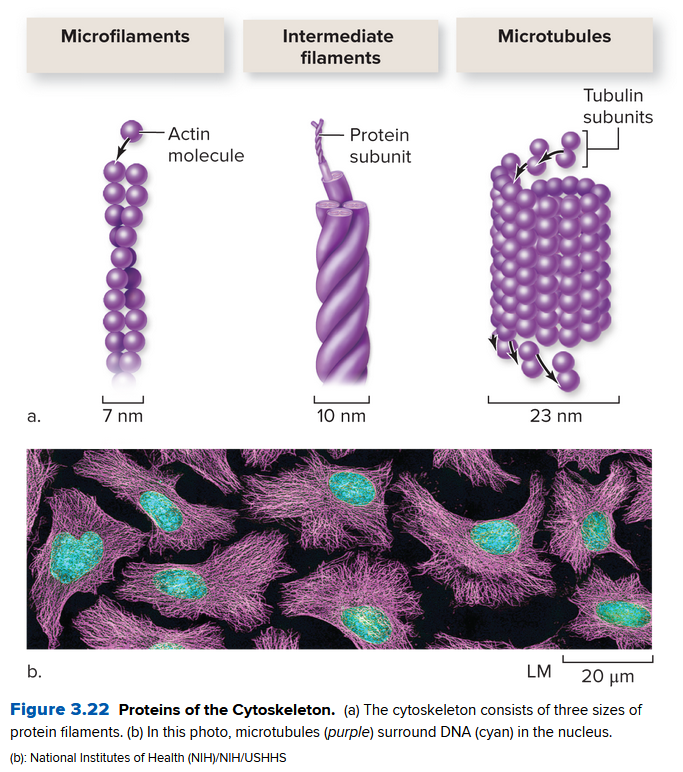
Ch 3.5: The Cytoskeleton Supports Eukaryotic Cells
The cytosol of a eukaryotic cells has a cytoskeleton, a network of protein "tracks" and tubules. It:
- is a transportation system
- provides physical support to maintain a 3D shape.
- helps cell division and combining
- allows the cell to move
Three main parts:
- microfilaments: a long rod of the protein actin
- intermediate filaments: form an internal scaffold in the cytosol and resist mechanical stress. Help bind cells together
- microtubules: composed of a protein (tubulin) formed into a tube structure. Substances in the cell move via these.
In animal cells, structures called centrosomes organize microtubules. They contain two centrioles that indirectly produce some cells that move: cilia and flagella.
Cilia are short, numerous extensions resembles a fringe. Unlike cilia, flagella occur singly or in pairs, and a flagellum is much longer.
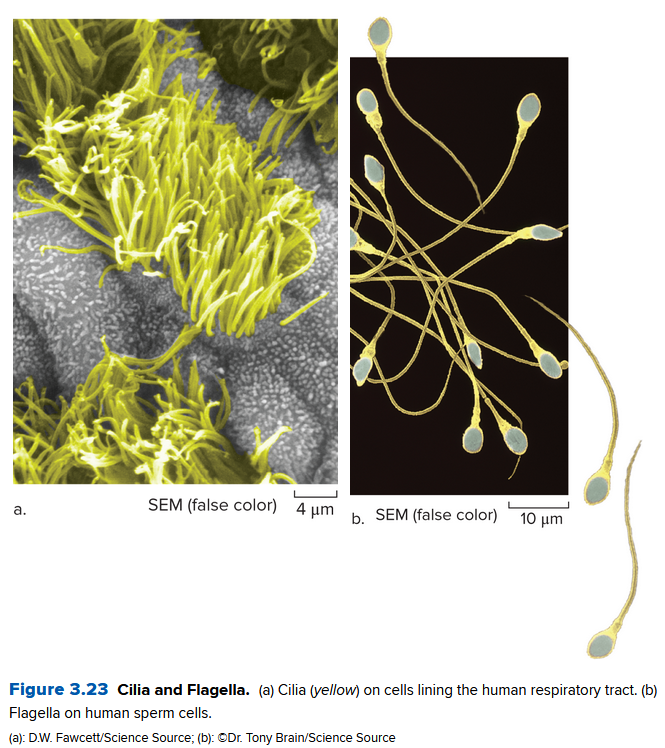
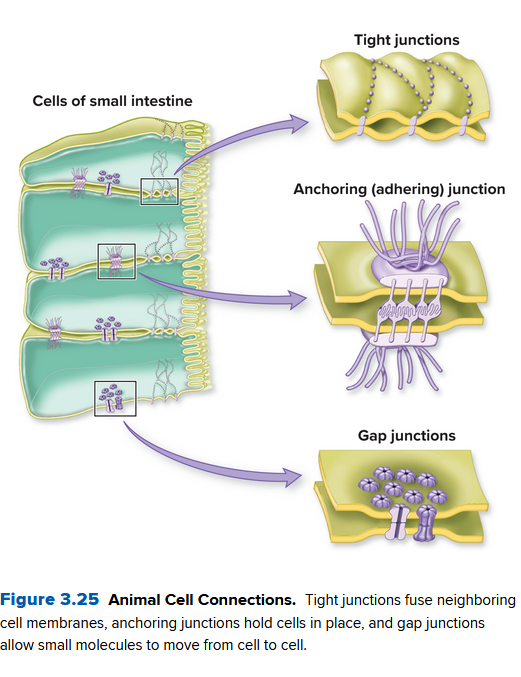
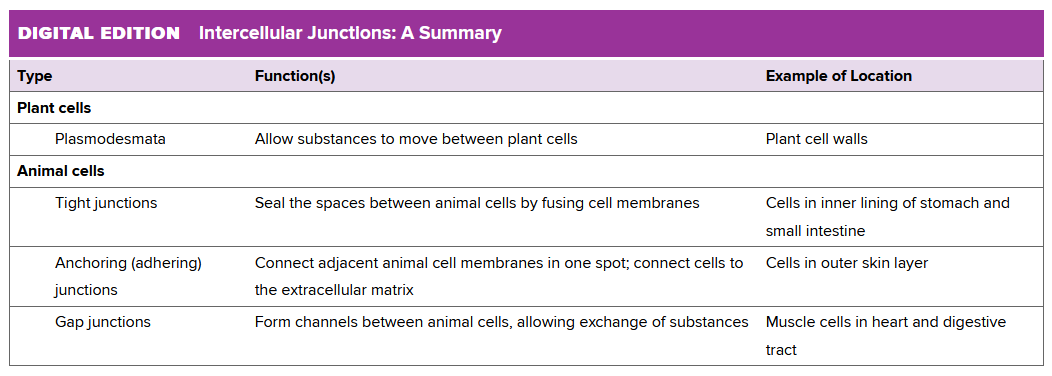
Ch 3.6: Cells Stick Together and Communicate with One Another
Plant cells communicate through their cell wall via channels called Palsmodesmata. They are tunnels in the cell wall, so material can move through via a thin strand of cytoplasm.
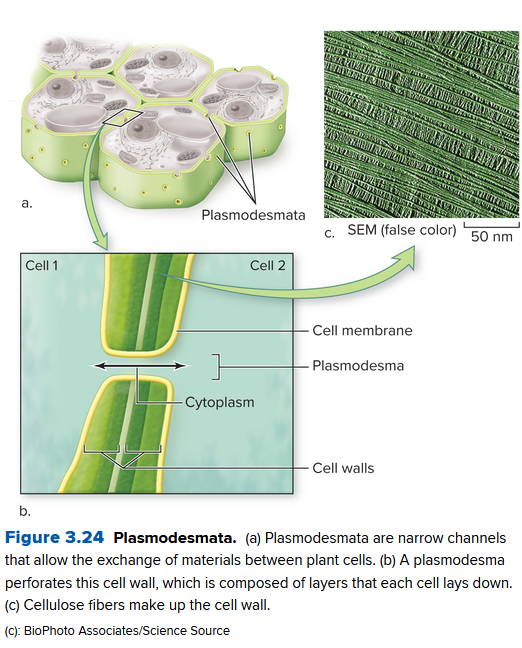
Animals don't have a cell wall. Instead, they connect tissues (matrices) together in different ways:
- tight junctions: fusing animal cells together to form an impermeable barrier
- anchoring (or adhering) junction: connecting an animal cell to its neighbors or to the extracellular matrix
- gap junction: protein channel that links the cytoplasm of adjacent animal cells, allowing the exchange of ions, nutrients, and other small molecules.