Lecture 4 - (missed)
See Ch. 4-6, or the slides [[02 Lecture Slides Chs 4-6.pdf]].
Cells and Energy
What is Energy?
Just as in any field, in biology energy is converted from one form to another.
![[02 Lecture Slides Chs 4-6.pdf#page=3]]
The most important energy transformations are photosynthesis and cellular respiration.
Chemical Reactions Sustain Life
In biochemistry, metabolism is all the chemical reactions, both anabolic (where you build up structures) and catabolic (breaking down biological molecules).
Both photosynthesis and cellular respiration only occur because reactions are coupled to other reactions.

But rather than an instantaneous release, energy is liberated via successive redox reactions in electron transport chains. To illustrate, if you were to release all of the energy in a burger, it would literally explode. It's a tremendous amount of energy. When cutting ATP, as another example, you release 4kJ, so 4,000 punches to the face. Humans, at the lowest resting state, uses 4 billion ATP molecules per minute!
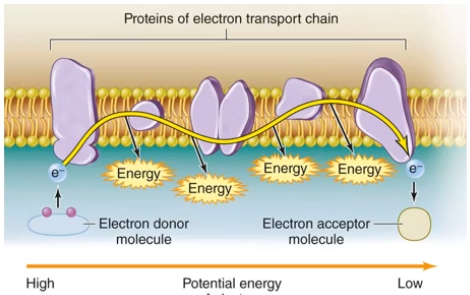
So, instead of breaking the energy down all at once, it's better to have proteins in a phospholipid bilayer. The lipid allows for a hydrophobic environment, to insulate it. The electron donor molecule will get oxidized (via a series of redox reactions) and then creates energy via a current. This releases needed energy, usually helping with other proteins to create ATP.
Much of this energy is stored as a chemical potential energy via adenosine tri-phosphate (ATP).
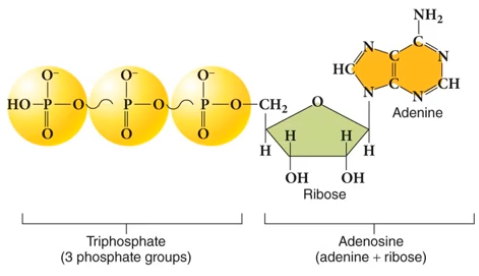
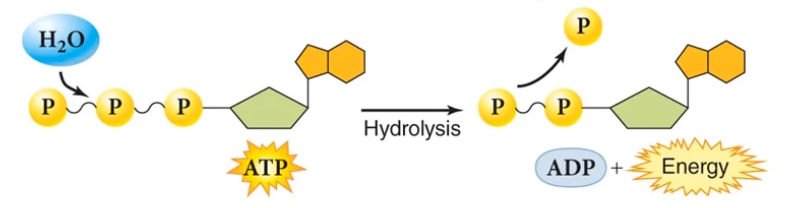
ATP is excellent for temporary energy storage. It's essentially a compressed spring, but as a result, it's very volatile.
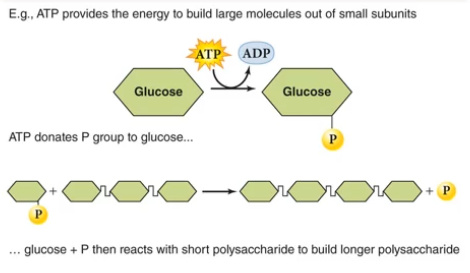
ATP is widely used since phosphate groups are added/removed to other molecules (for instance, fructose or other sugars) to create a chain, or to split the chain.
Organisms require hug amounts of ATP; an adult uses 2 billion per minute to stay alive. ATP is the driving force behind many enzymatic reactions. Enzymes are protein biocatalysts that reduce the activation energy for biochemical reactions. Lowering the activations energy "speeds up" the reactinos because more can occur per given amount of energy.
Enzymes lower activation energy by providing an active site in which substrates are bound and reactions can occur. Enzymes are highly regulated.
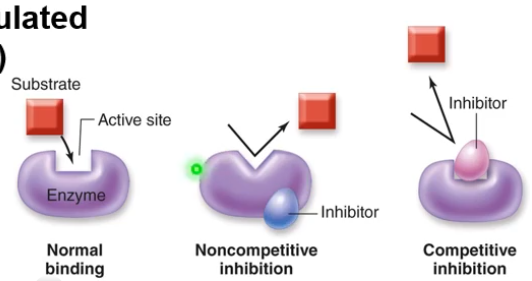
These are needed since, if left unchecked, the rate of chemical reactions may be too quick. Enzymes are sensitive to conditions in the cell, so since they are made of proteins they will denature and thus may start to "break" at a certain temperature.
The Cost of Membrane Transport
Membranes (remember, the phospholipid bilayer) are selectively permeable; essential to life.
They maintain concentration gradients through passive and active transport; also uses vesicles and other means:
- Passive transport: Net movement is down concentration gradient; no energy is required
- Simple Diffusion: Substance moves across membrane without assistance of transport proteins (movement is slowed by the membrane, just impeded slightly)
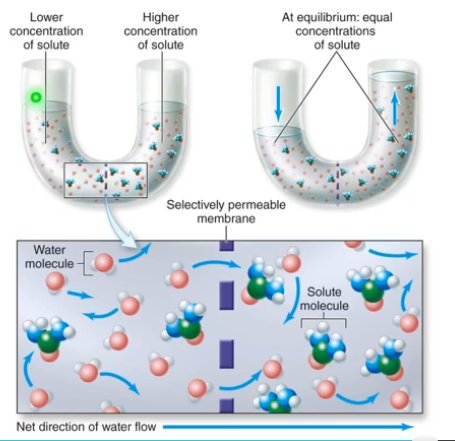
- Osmosis: Water diffuses across a selectively permeable membrane
- The water will go across the membrane where the solute can't. From the area of low concentration to high concentration.
- Helps generate osmotic pressure
- Facilitated diffusion: Substance moves across membrane with assistance of transport proteins (a channel allows molecules to primarily go through here, in controlled quantities. This can even generate potential energy too like a dam)
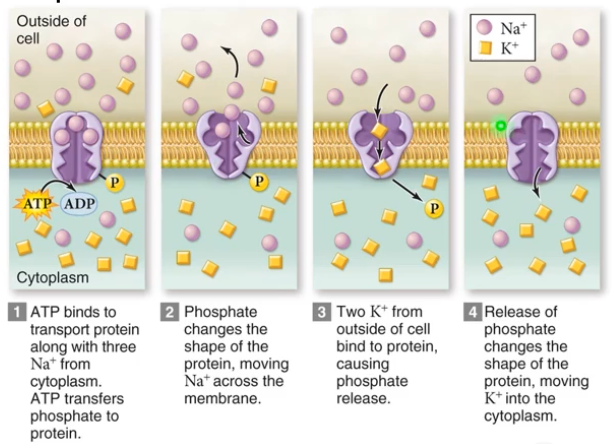
- Active Transport: requires energy input because it creates and maintains a concentration gradient
- Gradients represent Potential Energy so ATP is used to maintain them.
- As an example is the Sodium, Potassium Pump
One last thing to mention is that we are talking primarily smaller molecules. However, larger molecules can enter and leave the cell membrane via endocytosis (the molecules enter the cell), phagocytosis (cellular eating, cells eat other cells), and exocytosis (the molecules exit via a vesicle) to move large molecules in and out of the cell.
Photosynthesis
Inside a chloroplast, photosynthesis occurs in 2 stages, the light reactions and the carbon reactions. Each chloroplast has a granum, where a lot are in there.
In the light reactions,
To compare, lights in a room have a high potential energy difference in the electronics. This electrical energy excited electrons in atoms of the light. This makes the electron change energy levels and "teleport", emitting a photon of light on the transition, creating the light we want.
Photosynthesis is the opposite. The light ejects and electron, creating a potential difference in the membrane to drive other chemical reactions.
![[02 Lecture Slides Chs 4-6.pdf#page=15]]
Notice the steps:
- It all starts at photosystem II (why the name, we discovered it second). There's chloryphyll a here. Light hits this, causing it to eject electrons.
- To replace the two electrons,
is split from the electrons to create the and . - Electrons travel due to a potential difference, creating energy that is supplied to proteins on the way. This pumps molecules against the concentration (active transport).
- Protons pumped in by the movement of the electrons drives an enzyme called ATP synthase that converts ADP and P to ATP (using these protons) (this is facilitated diffusion).
- In photosystem I, the same thing happens. Light enters and hits a different chloryphyll b that raises the potential of the electron.
- NADP+ is turned into NADPH via this electron transport chain again
Note that
Know that Photosystem II comes before Photosystem I
The carbon reactions (known as the Calvin cycle) produce the carbohydrates. We haven't used the
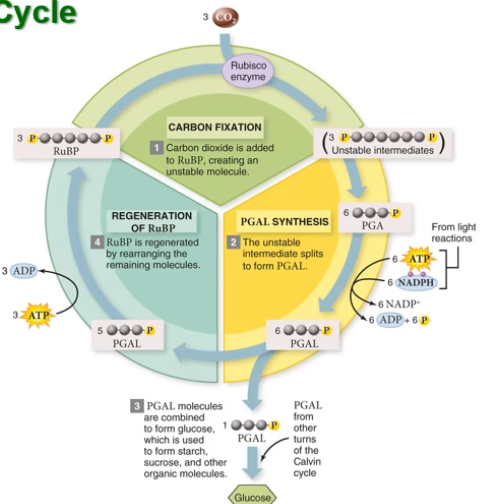
You don't really need to know the process in detail. But just know that:
- Carbon is fixed from
into organic molecules like glucose and sucrose - Carbs can then be used in several ways
- Cellular respiration
- Anabolism
- Energy Storage (eg: starch or sucrose)
How Cells Release Energy
We know energy in the form of ATP (or NADPH) is required for life, it's essential for cellular biochemistry. All living cells need ATP, but they don't produce it in the same way:
- Photosynthesis in plants
- Aerobic cellular respiration: cells use
and glucose to generate ATP. - Fermentation: ATP generation without
anaerobic respiration common in microbes.
Aerobic respiration is the reverse of photosynthesis:
In the mitochondria we take the glucose and oxygen to burn these into the products you see above. Cellular respiration includes three main processes to use the energy stored in food to make ATP. These small successive reactions carefully break down this energy as to not break it all at once. The three paths are:
- Glycolysis:
- Always in the cytoplasm, right outside the mitochondria
- The Krebs Cycle
- In mitochondria if possible
- Electron Transport
- In the mitochondria if possible
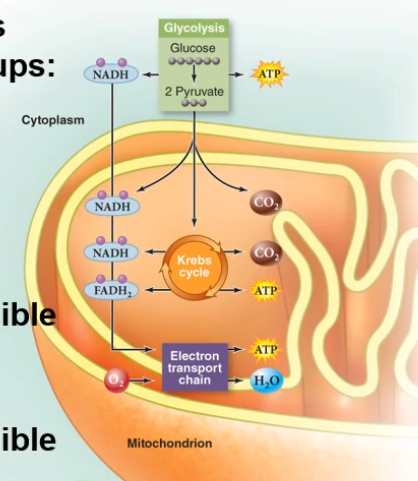
- In the mitochondria if possible
In bacteria and archaea, they use cytoplasm and membrane. But in eukaryotic cells, KC and ET occur in mitochondria
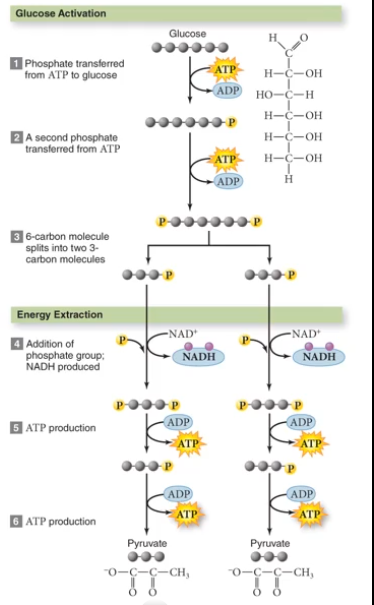
Glycolysis: Breaks Down Glucose
Glycolysis means "sugar splitting", and requires oxygen. It only nest 2 ATP and 2 NADH. We're trying to make 36, so this is out in terms of the process that produces the most ATP.
Aerobic Respiration
Aerobic respirations yields much more ATP than glycolysis alone. This is just the use of glucose; we eat other foods like fats and protein. Look at the figure to see how these other foods are broken down:
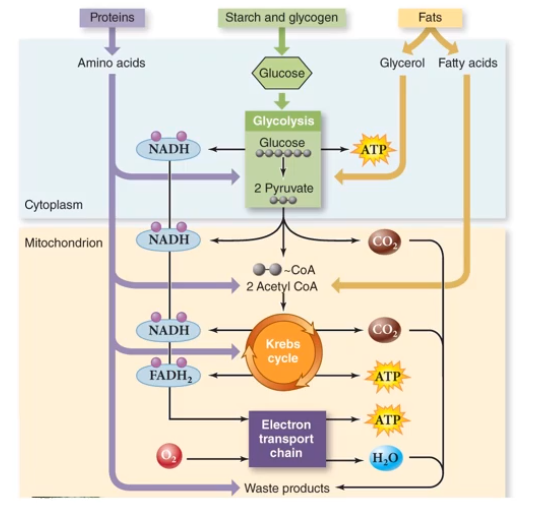
Notice if you consume fats, there is no line to waste products (hence why it's hard to remove it from your body).