Lecture 3 - Finishing the Cells
We finish talking about the major biological molecules, from:
- Nucleic Acids
- Proteins
- Carbohydrates
- Lipids
Lipids
All lipids are hydrophobic and do not dissolve in water (some are completely indissolvable). Lipids are also known as fats. But some are used in building membranes and chemical signaling like cholesterol and testosterone.
Things like cholesterol are used, for instance, to neutralize contents that leave the stomach, covered in stomach acid (which is akin to
Proteins
Proteins are complex and highly versatile. Membrane proteins, hemoglobin, keratin, polymerase, antibodies, enzymes, and so on are all proteins.
For instance, should you eat a lot of vitamin C? Some vitamins are fat soluable and some are not. Vitamin C will be peed out in excess. But some vitamins in multi-vitamins are fat-soluable, so they could accumulate in your body. And bacteria could potentially feast on them, thus making you more prone to infection.
But some proteins like feratin help encase certain minerals like Iron to keep away the bacteria. Ovalbumin helps do the same things, and are in eggs. Hence, when you have eggs for a long time, the egg protein is usually broken and thus bacteria feast on the eggs and then make sulfur from their own digestion.
The most important function of proteins is to conduct chemical reactions. That's it. Enzymes catalyze most chemical reactions within the body. Chemical reactions have a very, very, very low chance of happening on a whim. But enzymes help force these reactions have, with enough products available.
A protein is a chain of amino acids, linked together by peptide bonds to form one or more polypeptides. Amino acids are the monomers, polypeptides are the polymers.
Here, the amino acid is made of an animo group, carboxyl group, and a variable R-group.
There's an N-terminis that terminates the protein on one end via the amino group. Similarly, there's an C-terminis where the carbooxyl group terminates the other end.
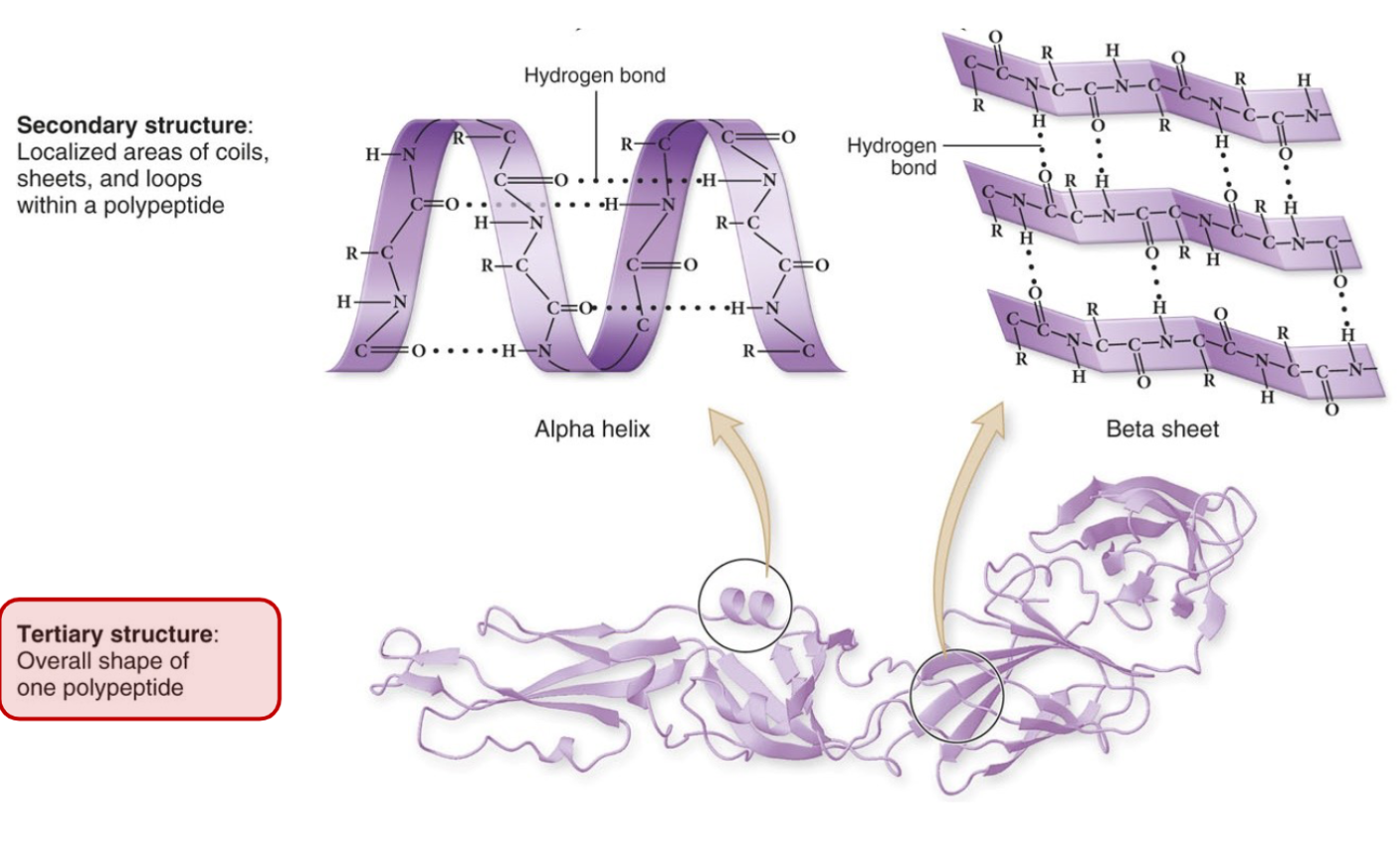
We get a primary structure from this single chain. The secondary structure comes from the sheets that occur, or the loops that occur due to the
A protein is a chain of amino acids linked together by peptide bonds to form one or more polypeptides. We can make some of the 20 naturally occurring amino acids from scratch but we get half - the essential amino acids - from the proteins we eat.
Protein shape is critical for function. There's a protein that's non-polar that lines the surface of your lungs. Without the protein, it'd just bounce off your lung and thus not get diffused in. You need to the protein to take oxygen out of the air. Specifically, the shape of this protein is a very long shape, that helps "catch" these non-polar
Most proteins end at tertiary structure:
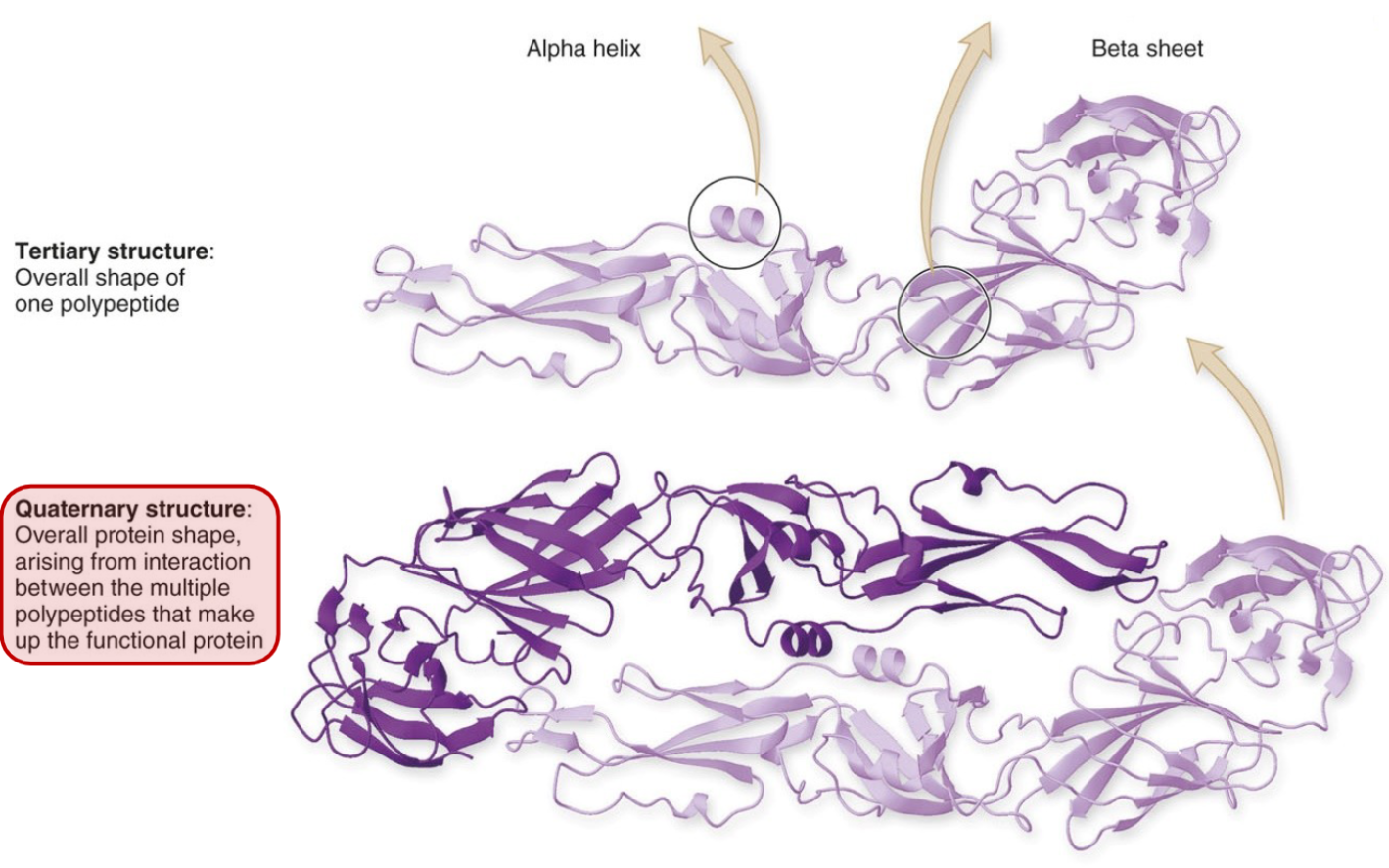
But if you have 2 or more polypeptides, you have a quaternary structure, of multiple tertiary structure.
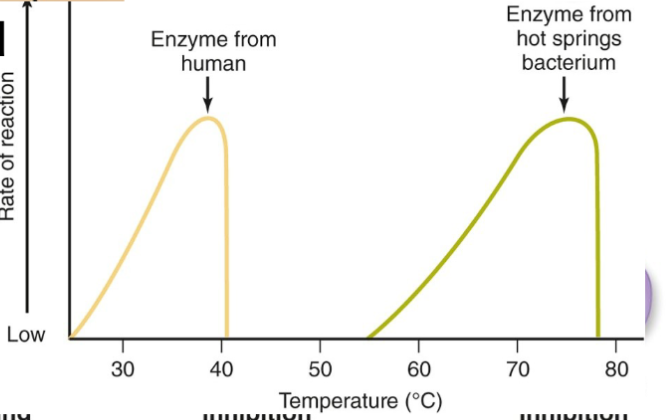
Proteins are vulnerable to heat, pH, salts, etc. that can denature them and render them non-functional.
There's actually a way to reverse the process of denaturing. This is useful since we can then get a productive protein back, even after burning it or something similar.
Nucleic Acids
Nucleic monomers make up long nucleic acid polymers like deoxyribonucleic acid (DNA) or ribonucleic acid (RNA). Each of these has:
- a sugar (pentagon/ring structure).
- a nitrogenous base
- a phosphate group
The difference between the bases (A, C, G, T in DNA, A, C, G, U in RNA). Dehydration synthesis and hydrolysis bring together and separate the parts of these nucleic monomers together and apart. The phosphate group's OH and H are these connector molecules.
DNA is far more stable than RNA because the OH in the phosphate group wants to react with the H in deoxyribose (on the bottom), thus making their formation far easier. Ribose is missing this oxygen.
Cell Structure (Units of Life)
Organisms are made up of 1+ cells, the cell is the fundamental unit of life, and cells come from preexisting cells. These cells are small.
Cells all have certain features in common:
- DNA and RNA
- Ribosomes
- Proteins
- Cytosol/cytoplasm
- Cell Membrane
- Membranes are central to life
- They make it possible to and maintain the exchange of nutrients into and out of the cell
- Also control the ratio of surface area of volume.
We can page the slides:
![[01 Lecture Slides Chs 1-3.pdf#page=63]]
Cells and Energy
Energy is the capacity to do work. All types of energy are either potential energy and kinetic energy. In biology, a common method of potential energy is a concentration gradient, where a higher concentration of ions on one side of a membrane creates a potential difference, where proteins here are driving chemical reactions by using that concentration gradient.
A 1kg fist, with 1 meter per second squared travelling at 1 meter, will gain 1J of energy. Hence, a punch is about 1 unit of energy.
We use calories (1 cal = 4.184J, the energy to heat up 1g of water 1 degree centigrade). Here 1 Calorie (nutritional calories) is 1kcal. Thus, 4.184kJ make up a nutritional calorie. The average person eats 2000 calories a day, so then 8.368MJ per day. That's 8 million punches needed per day.
An notice that, like octane that uses combusion (fuel and oxygen) to make energy, humans and any life does the same thing.
And just like in any field, energy transfer between different forms. The most bioefficient forms of algae that takes the energy from the sun are at an abyssmal 8%. However, a lot of energy is spent trying to compete with other organisms. Rain forests have plants that are fighting to get access to the sun. For us, maintaining body temperature takes most of our energy.
Most important energy transformations comes via photosynthesis and cellular respiration.
Chemical Reactions Sustain Life
In biochemistry, metabolism is all the chemical reactions in cells, with two categories, anabolic and catabolic. Here, photosynthesis is anabolic, and respiration is catabolic.
Both photosynthesis and cellular respiration only occur because reactions are coupled to their counterparts. Rather than an instantaneous release, energy is liberated via a successive redox reaction in electron transport chains.
You need to do this process in an electrically-neutral environment, so that you can control the reaction. As such, membrane internals are this environment, and thus are usually where respiration or photosynthesis occurs. Namely, the mitochondria will have a lot of these membranes as a result.
Much of this energy is stored as chemical potential energy in the bonds of adenosine tri-phosphate (ATP).
The electrical properties of this molecule makes it create a great negative potential, allowing it to hold a great amount of energy. ATP is great for portablility in terms of energy (it's a good spring) and thus is a great temporary storage of energy. However, ATP is very unstable, due to this feature of it.
ATP thus is great for temporary energy storage, but not long term. Cells use ATP as energy by phosphorylating molecules. Organisms require huge amounts of ATP. For context, and adult uses 2 billion per minute to stay alive. ATP is the driving force behind many enzymatic reactions. Enzymes are protein biocatalysts that reduce the activation energy for biochemical reactions.
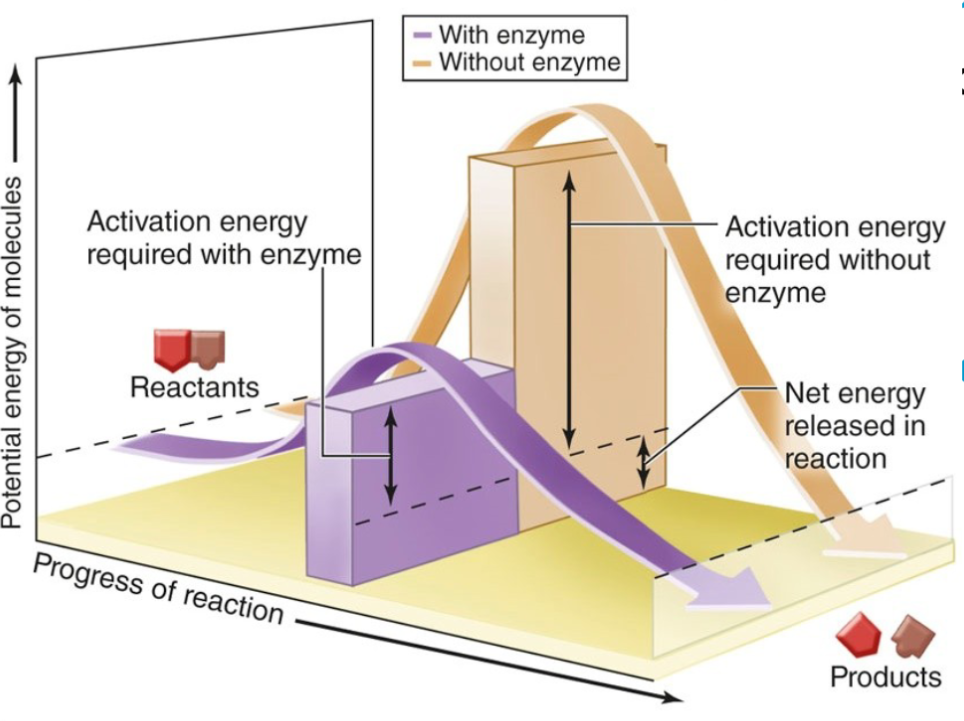
Enzymes are protein biocatalysts that reduce the activation energy for biochemical reactions. Lowering the activation energy "speeds up" the reactions because more can occur per a given amount of energy. Enzymes lower the activation energy by providing an active site in which substrates are bound and reactions can occur. Enzymes are highly regulared (ie: have negative feedback).
Enzymes are made of protein, so they will denature at high temperatures: