Lecture 2 - What is Life (cont.), Taxonomy
From last time we had the 5 qualities that made life:
- Life is organized
- Life requires energy
- Life maintains internal consistency (via: homeostatis)
- Life reproduces, grow and develops
- Life evolves.
We continue our exploration.
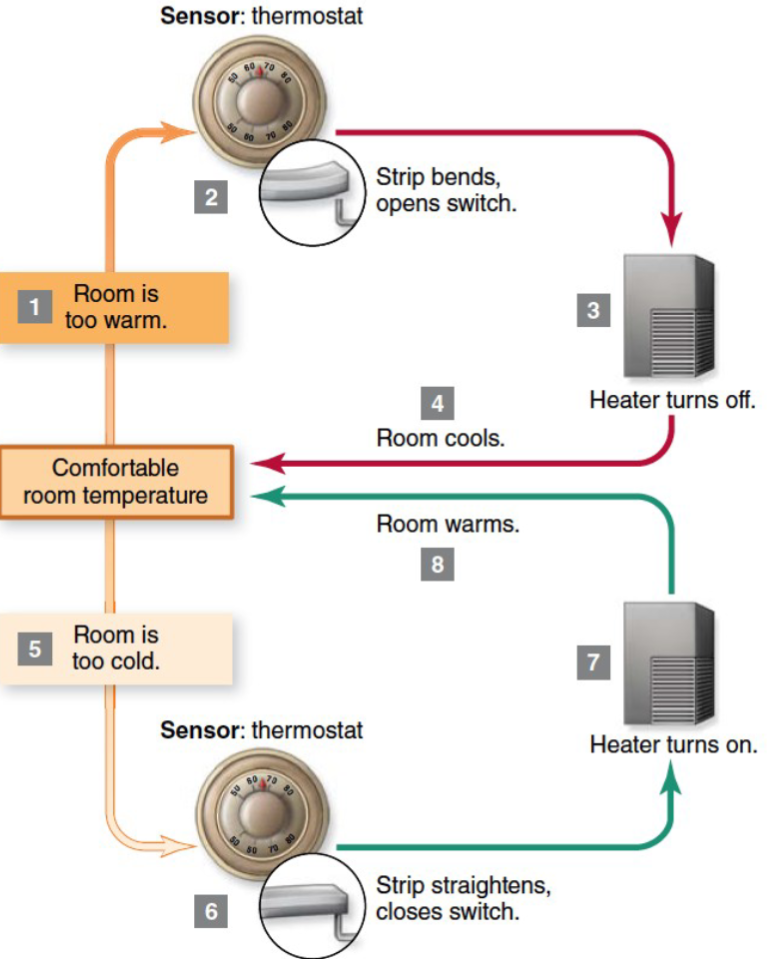
(3) Homeostatis
Life has mechanisms that help create internal stability:
(4) Reproduction
Some organisms reproduce asexually, others reproduce sexually and combine characteristics.
A vast majority of these organisms will be identical, and thus are all susceptible to the same genocidal viruses or other predators. As such, to avoid this:
- asexual organisms will just produce a lot of offspring (playing the numbers game)
- Pros: don't have to find other mates
- Cons: they have to make so many offspring
- sexual organisms will mix DNA/genetic material
- Fun fact: incest is bad lol.
- Pros: we can evolve our genes to have better traits through generations. Lucky genes will survive.
- Furthermore, since there are so many genes, each human is very unique. There's continuous mixing.
- You also only just need at least one offspring (technically two, but once you got one you're pretty set).
- Cons: you're playing the lottery to see if the offspring
The fungus below is asexual. The plants and animals are not.

(5) Life Evolves
In biology, adaptation occurs through mutation and natural selection; in engineering you make a mock up and troubleshoot.
You build up mutations over time, no matter what (ie: aging). Evolutionary time is a long time.
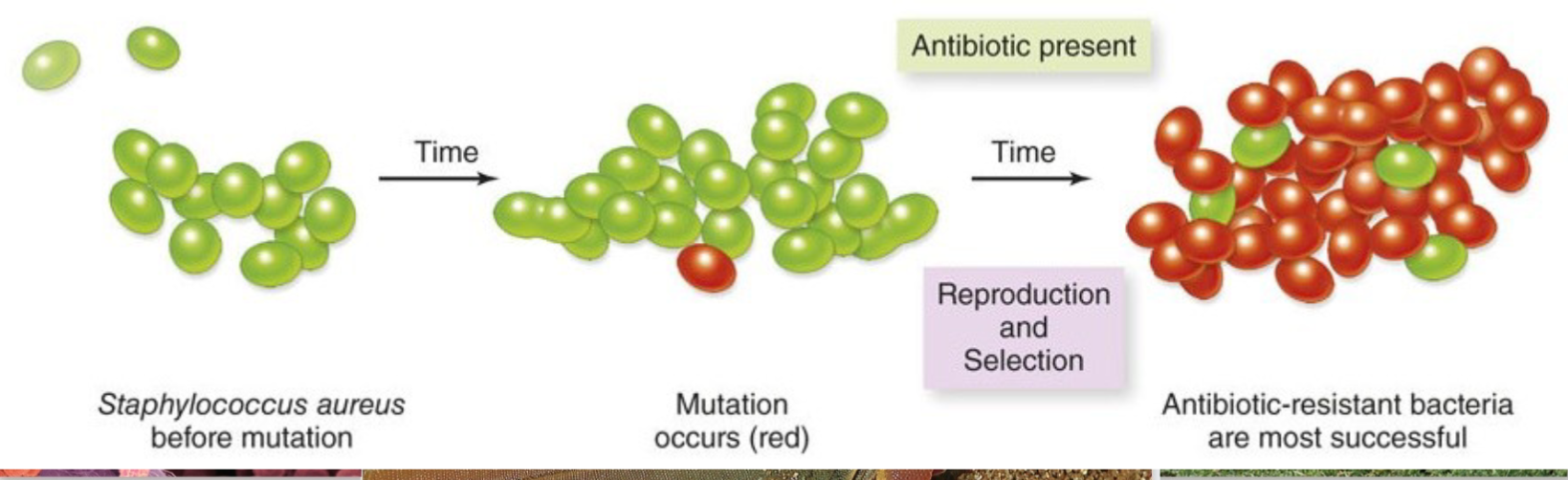
For example, if you take antibiotics, you automatically kill the less resistance bacteria. Then, you immune system can take care of the ones that have resistance manually. But if you stop taking antibiotics, you allow the mutations to live for more time, thus making it harder in the long run:
Taxonomy: Classifying Life
Now let's classify life. We can name and classify organisms via taxonomy. We can use genetics to see how similar and different things are.
Living organisms are classified into one of three domains, and then further classified into genus and species.
Way at the top there are three domains:
- Domain Bacteria
- Domain Archaea
- Domain Eukarya
For example, bacteria only have one membrane, while we humans have multiple membranes. Archaea are very similar to bacteria in some ways, and Eukarya in others. In general, anything not in the Domain Eukarya are Prokaryotes.
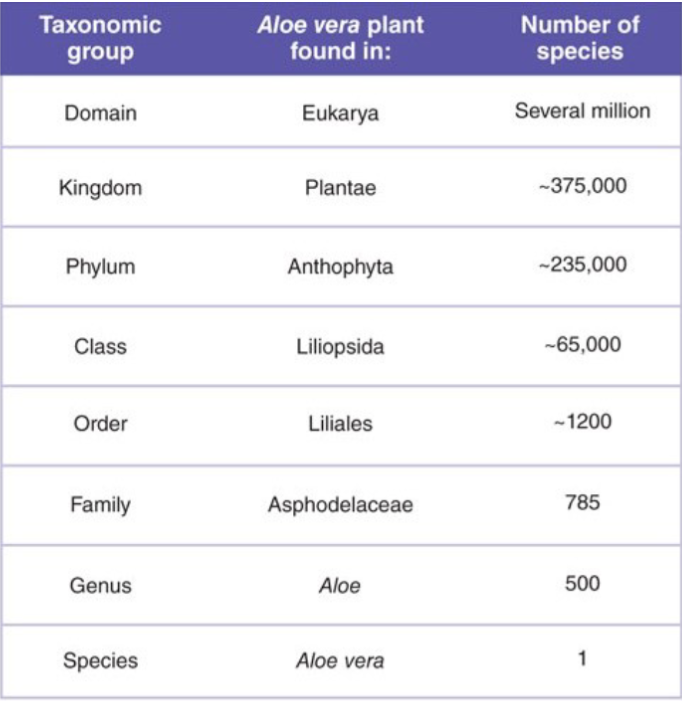
For example, Homo Sapiens have the genus of homo, and the species of sapiens. Aloe vera is the weird one since the genus is aloe, and the species is aloe vera. We use genus and species to talk primarily about groups of bacteria.
Review of The Chemistry of Life
Like all matter, organisms are composed of elements. But not all elements are in living matter. Only about 25 elements are essential to life. And of these, only the bulk elements make up the vast majority of every living cells. Trace elements are sparse:
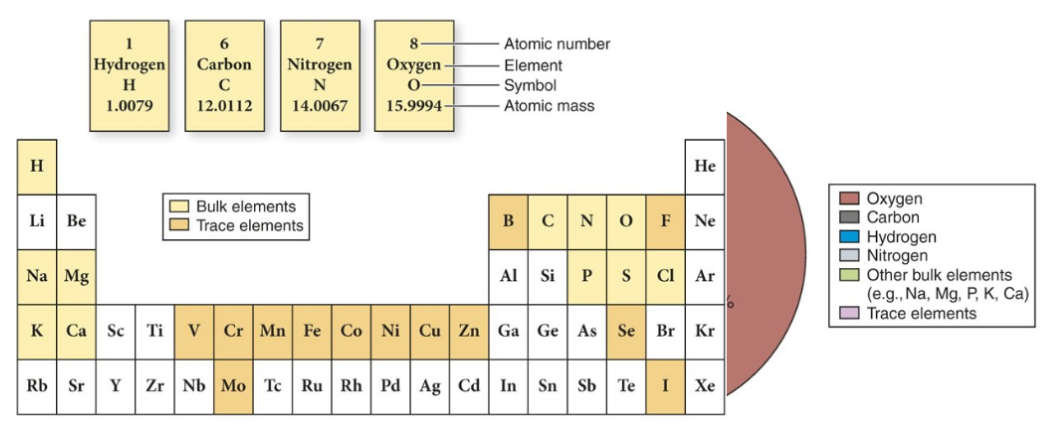
SCHNOPS are the most common elements. See the right side of the graph to see the most common elements by mass on the top right.
What is the most abundant element in the human body by mass?
Answer: oxygen
Why is oxygen so common? It's because we're made of a whole bunch of water, and for H2O there's more mass of oxygen than hydrogen per molecule.
Like all matter, living matter contains atoms of these essential elements chemically bonded to one another. There's just covalent, ionic, and metallic bonds. The strong bonds in the biological world are mostly covalent bonds via the SCHNOPS elements. They aren't metals, and aren't really ionic (or are ionic in rarer, less massive, elements).
Each molecule or compound formed isn't alive (obviously) but they are utilized to maintain the life of an organism. Biologists like to consider how to use biologic covalent bonds to create more biological material science.
For example, we can use the power of bonds to store energy. You can either break the bond to release energy. When you burn ethenol, you don't release a lot of energy, because of the single Carbon bonds of it. But if you have a double bond like in ethylene, then you release more energy when you break that bond.
Plants/fruits make ethylene gas, which ripen the fruit. You pick the ripe fruit to have a tougher fruit for transport, then gas the fruit with ethylene to ripen them up, then they go to the store.
A triple bond like that found in Acetylene has a lot of energy! Almost too much for the purposes of biology. But it shows that bonds are a place to store a lot of energy.
But look at the structure of caffeine.
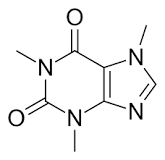
It doesn't really have that much energy. It relies on essentially increasing the clock cycle of the body, but it then burns more energy in the same period of time.
Furthermore, water is essential to life. Water has extraordinary properties because it's polar and can hydrogen-bond. It also is cohesive.
It's also a solvent. Namely, water surrounds NaCl and breaks the ionic bonds, dissociating, namely because enough H-bonds can overcome these ionic bonds, thus creating the dissociating.
We use water to regulate temperature via homeostasis. You're made of a lot of water which helps stabalize your temperature (as it takes a while to heat the water up).
Water also expands as it freezes. And that's essential to life, because the top layer of a river freezes, which insulates the really cold air up top from the warmer water underneath.
It also participates in most of life's chemical reactions. It participates in things like photosynthesis, and in sugar burning.
The Organic Chemistry of Life
In addition to water, carbon-based organic molecules are essential to life. They are often polymers of monomeric subunits.
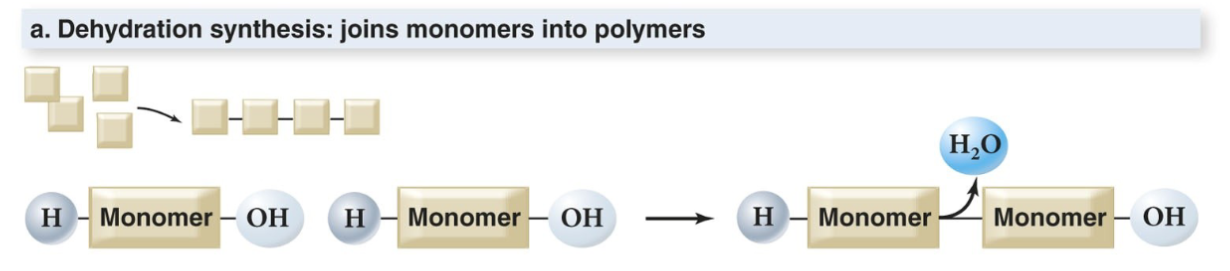
And the reverse process is hydrolysis. Capillary action is also an action of water.
Living organisms utilize polymers to make more complex macromolecules with specialized functions:
- Carbohydrates: energy storage, structure
- Lipids: membranes, energy storage, signaling
- Proteins: structure, locomotion, transport, storage, catalysis, +++
- Nucleic Acids: information storage and transmission
We'll go through each one.
Carbohydrates
Carbohydrates are sugars; simple sugars are monosaccharides. Ex: ribose, glucose, fructose.
They are joined together and form disaccharides.
What is the most abundant monosaccharide on the planet? What about disaccharide?
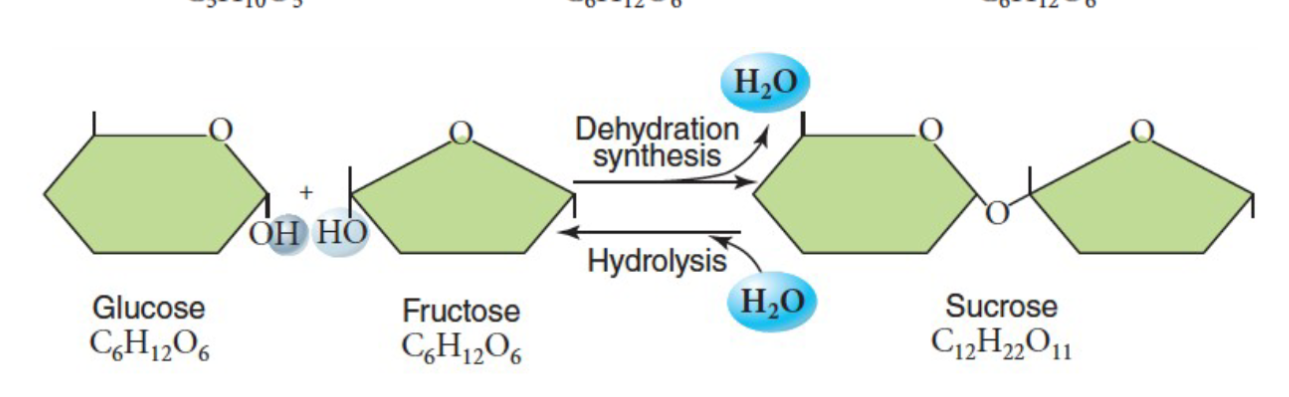
Notice the monomer structure that we saw before.
Complexity grows from there giving polysaccharides.
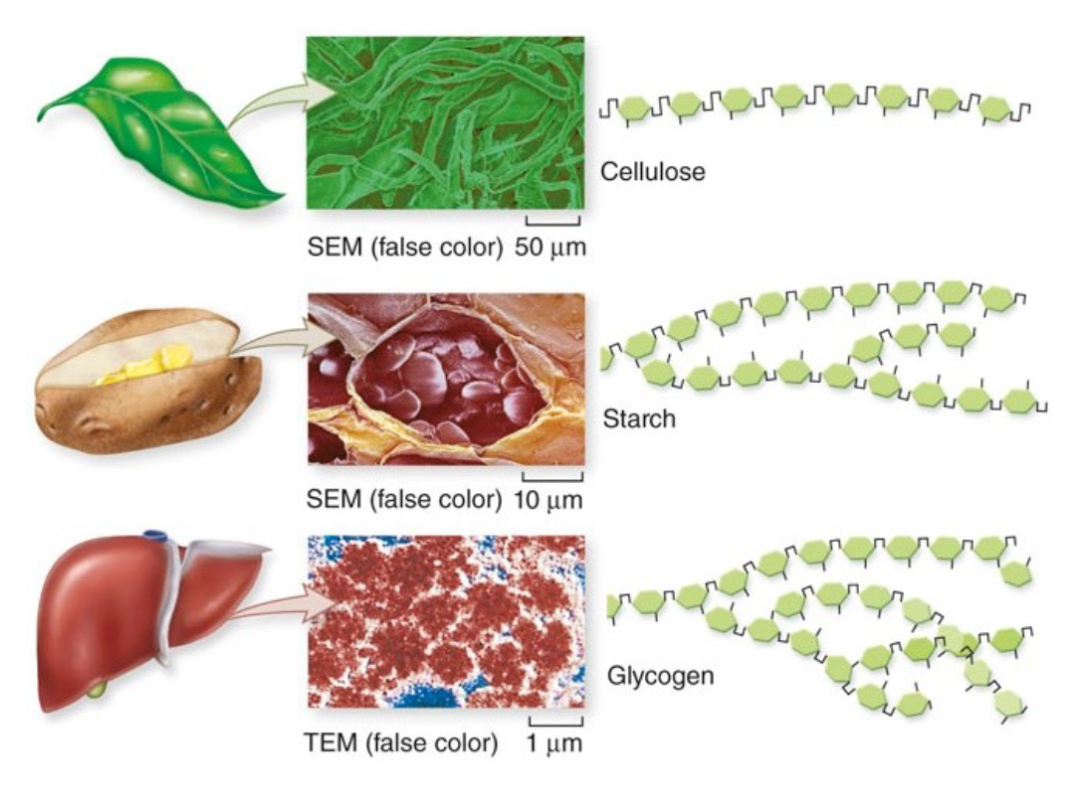
Plants use cellulose for structure, starch for energy storage, and glycogen for a mix of both.
We'll cover the rest next time...